The Delivery of the Novel Drug ‘Halicin’ Using Electrospun Fibers for the Treatment of Pressure Ulcer against Pathogenic Bacteria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Electrospinning Solution
2.3. Preparation of Monoaxial Fibers
2.4. Scanning Electron Microscopy (SEM)
2.5. Fourier-Transform Infrared Spectroscopy (FTIR)
2.6. X-Ray Diffraction (XRD)
2.7. Ultraviolet/Visible Analysis (UV/Vis)
2.8. Drug Loading (DL), Entrapment Efficiency (EE%), and Fiber Yield (Y)
2.9. Drug Release Profile
2.10. Bacterial Suspensions Preparation for Microbiological Testing
2.11. The Minimum Inhibitory Concentration (MIC)
2.12. Antibacterial Zone of Inhibition Assay
2.13. Statistical Analysis
3. Results & Discussion
3.1. Morphological Characteristics of the Electrospun Fibers
3.2. Fourier-Transform Infrared Spectroscopy (FTIR)
3.3. X-Ray Diffraction (XRD)
3.4. UV Standard Curve
3.5. Drug Loading (DL), Encapsulation Efficiency (EE%), and Fiber Yield (Y)
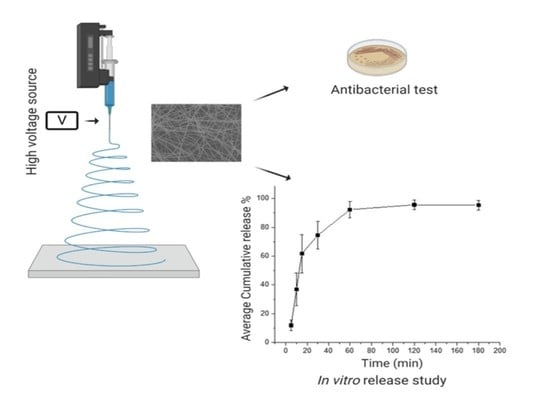
3.6. Drug Release Profile
3.7. Antibacterial Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gebhardt, K. Pressure ulcer prevention. Part 1. Causes of pressure ulcers. Nurs. Times 2002, 98, 41–44. Available online: http://www.ncbi.nlm.nih.gov/pubmed/11933809 (accessed on 28 August 2020). [PubMed]
- Thomas, D.R. Prevention and Treatment of Pressure Ulcers. J. Am. Med Dir. Assoc. 2006, 7, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Gefen, A. Reswick and Rogers pressure-time curve for pressure ulcer risk. Part 1. Nurs. Stand. Royal Coll. Nurs. 2009, 23, 64. [Google Scholar] [CrossRef]
- Gefen, A. Reswick and Rogers pressure-time curve for pressure ulcer risk. Part 2. Nurs. Stand. 2009, 23, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Callam, M.J.; Ruckley, C.V.; Harper, D.R.; Dale, J.J. Chronic ulceration of the leg: Extent of the problem and provision of care. BMJ 1985, 290, 1855–1856. [Google Scholar] [CrossRef] [Green Version]
- Krouskop, T.A.; Reddy, N.P.; Spencer, W.A.; Secor, J.W. Mechanisms of decubitus ulcer formation—An hypothesis. Med. Hypotheses 1978, 4, 37–39. [Google Scholar] [CrossRef]
- Reddy, N.P.; Cochran, G.V.B.; Krouskop, T.A. Interstitial fluid flow as a factor in decubitus ulcer formation. J. Biomech. 1981, 14, 879–881. [Google Scholar] [CrossRef]
- Reddy, N.P.; Patel, K. A mathematical model of flow through the terminal lymphatics. Med. Eng. Phys. 1995, 17, 134–140. [Google Scholar] [CrossRef]
- Leblebici, B.; Turhan, N.; Adam, M.; Akman, M.N. Clinical and Epidemiologic Evaluation of Pressure Ulcers in Patients at a University Hospital in Turkey. J. Wound Ostomy Cont. Nurs. 2007, 34, 407–411. [Google Scholar] [CrossRef]
- Asmatulu, R.; Khan, W.S. Synthesis and Applications of Electrospun Nanofibers. Synth. Appl. Electrospun Nanofibers 2019. [Google Scholar] [CrossRef]
- Tawfik, E.A.; Craig, D.Q.M.; Barker, S.A. Dual drug-loaded coaxial nanofibers for the treatment of corneal abrasion. Int. J. Pharm. 2020, 581, 119296. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Zhang, X.; Liu, H.; Xu, P.; Doulathunnisa; Teng, G.-J.; Xiao, Z. Neuronally differentiated adipose-derived stem cells and aligned PHBV nanofiber nerve scaffolds promote sciatic nerve regeneration. Biochem. Biophys. Res. Commun. 2017, 489, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Hsieh, P.C.H.; Takahashi, T.; Song, Q.; Zhang, S.; Kamm, R.D.; Grodzinsky, A.J.; Anversa, P.; Lee, R.T. Local myocardial insulin-like growth factor 1 (IGF-1) delivery with biotinylated peptide nanofibers improves cell therapy for myocardial infarction. Proc. Natl. Acad. Sci. USA 2006, 103, 8155–8160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noh, K.H.; Park, Y.M.; Kim, H.S.; Kang, T.H.; Song, K.-H.; Lee, Y.-H.; Byeon, Y.; Jeon, H.N.; Jung, I.D.; Shin, B.C.; et al. GM-CSF-loaded chitosan hydrogel as an immunoadjuvant enhances antigen-specific immune responses with reduced toxicity. BMC Immunol. 2014, 15, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Olvera, D.; Sathy, B.N.; Carroll, S.F.; Kelly, D.J. Modulating microfibrillar alignment and growth factor stimulation to regulate mesenchymal stem cell differentiation. Acta Biomater. 2017, 64, 148–160. [Google Scholar] [CrossRef]
- Sundaramurthi, D.; Krishnan, U.M.; Sethuraman, S. Electrospun Nanofibers as Scaffolds for Skin Tissue Engineering. Polym. Rev. 2014, 54, 348–376. [Google Scholar] [CrossRef]
- Huang, C.; Wang, S.; Qiu, L.; Ke, Q.; Zhai, W.; Mo, X. Heparin Loading and Pre-endothelialization in Enhancing the Patency Rate of Electrospun Small-Diameter Vascular Grafts in a Canine Model. ACS Appl. Mater. Interfaces 2013, 5, 2220–2226. [Google Scholar] [CrossRef]
- Lin, X.; Tang, D.; Du, H. Self-assembly and controlled release behaviour of the water-insoluble drug nifedipine from electrospun PCL-based polyurethane nanofibres. J. Pharm. Pharmacol. 2013, 65, 673–681. [Google Scholar] [CrossRef]
- Zahedi, P.; Rezaeian, I.; Jafari, S.H. In vitro and in vivo evaluations of phenytoin sodium-loaded electrospun PVA, PCL, and their hybrid nanofibrous mats for use as active wound dressings. J. Mater. Sci. 2012, 48, 3147–3159. [Google Scholar] [CrossRef]
- Shababdoust, A.; Ehsani, M.; Shokrollahi, P.; Zandi, M. Fabrication of curcumin-loaded electrospun nanofiberous polyurethanes with anti-bacterial activity. Prog. Biomater. 2018, 7, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Rahman, S.; Carter, P.; Bhattarai, N. Aloe Vera for Tissue Engineering Applications. J. Funct. Biomater. 2017, 8, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berechet, M.D.; Gaidau, C.; Miletić, A.; Pilic, B.; Rapa, M.; Stanca, M.; Ditu, L.M.; Constantinescu, R.; Lazea-Stoyanova, A. Bioactive Properties of Nanofibres Based on Concentrated Collagen Hydrolysate Loaded with Thyme and Oregano Essential Oils. Materials 2020, 13, 1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramakrishna, S.; Zamani, M.; Prabhakaran, M.P. Advances in drug delivery via electrospun and electrosprayed nanomaterials. Int. J. Nanomed. 2013, 8, 2997–3017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fletcher, J. The benefits of using hydrocolloids. Nurs. Times 2003, 99, 57. [Google Scholar]
- Wasiak, J.; Cleland, H.; Campbell, F.; Spinks, A. Dressings for superficial and partial thickness burns. Cochrane Database Syst. Rev. 2013. [Google Scholar] [CrossRef] [Green Version]
- Molan, P.C. The evidence supporting the use of honey as a wound dressing. Int. J. Lower Extrem. Wounds 2006, 5, 40–54. [Google Scholar] [CrossRef] [Green Version]
- Leaper, D.J. Silver dressings: Their role in wound management. Int. Wound J. 2006, 3, 282–294. [Google Scholar] [CrossRef]
- Toy, L.W.; Macera, L. Evidence-based review of silver dressing use on chronic wounds. J. Am. Acad. Nurse Pr. 2011, 23, 183–192. [Google Scholar] [CrossRef]
- Martens, E.; Demain, A.L. The antibiotic resistance crisis, with a focus on the United States. J. Antibiot. 2017, 70, 520–526. [Google Scholar] [CrossRef] [Green Version]
- Center for Disease Control and Prevention. Antibiotic/Antimicrobial Resistance CDC; Center for Disease Control and Prevention: Atlanta, GA, USA, 2015. [CrossRef] [Green Version]
- Stokes, J.M.; Yang, K.; Swanson, K.; Jin, W.; Cubillos-Ruiz, A.; Donghia, N.M.; Macnair, C.R.; French, S.; Carfrae, L.A.; Bloom-Ackermann, Z.; et al. A Deep Learning Approach to Antibiotic Discovery. Cell 2020, 180, 688–702. [Google Scholar] [CrossRef] [Green Version]
- De, S.K.; Stebbins, J.L.; Chen, L.-H.; Riel-Mehan, M.; Machleidt, T.; Dahl, R.; Yuan, H.; Emdadi, A.; Barile, E.; Chen, V.; et al. Design, Synthesis, and Structure−Activity Relationship of Substrate Competitive, Selective, and in Vivo Active Triazole and Thiadiazole Inhibitors of the c-Jun N-Terminal Kinase. J. Med. Chem. 2009, 52, 1943–1952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, S.; Yu, L.-R.; Abdelmegeed, M.A.; Gao, Y.; Banerjee, A.; Song, B.-J. Critical role of c-jun N-terminal protein kinase in promoting mitochondrial dysfunction and acute liver injury. Redox Biol. 2015, 6, 552–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zgurskaya, H.I.; López, C.A.; Gnanakaran, S. Permeability Barrier of Gram-Negative Cell Envelopes and Approaches to Bypass It. ACS Infect. Dis. 2015, 1, 512–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.-J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Rogers, D.; Hahn, M. Extended-connectivity fingerprints. J. Chem. Inf. Model 2010, 50, 742–754. [Google Scholar] [CrossRef]
- Farha, M.A.; Brown, E.D. Unconventional screening approaches for antibiotic discovery. Ann. N. Y. Acad. Sci. 2015, 1354, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.R.; Raimi-Abraham, B.T.; Luo, C.J. Electrospinning fundamentals. Nanofib. Drug Deliv. 2018. [Google Scholar] [CrossRef] [Green Version]
- Kampshoff, F.; Willcox, M.D.; Dutta, D. A Pilot Study of the Synergy between Two Antimicrobial Peptides and Two Common Antibiotics. Antibiot. 2019, 8, 60. [Google Scholar] [CrossRef] [Green Version]
- Karatas, A.; Algan, A.H.; Pekel-Bayramgil, N.; Turhan, F.; Altanlar, N. Ofloxacin Loaded Electrospun Fibers for Ocular Drug Delivery: Effect of Formulation Variables on Fiber Morphology and Drug Release. Curr. Drug Deliv. 2016, 13, 433–443. [Google Scholar] [CrossRef]
- Gizaw, M.; Thompson, J.; Faglie, A.; Lee, S.-Y.; Neuenschwander, P.F.; Chou, S.-F. Electrospun Fibers as a Dressing Material for Drug and Biological Agent Delivery in Wound Healing Applications. Bioengineering 2018, 5, 9. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhou, S.; Gao, Y.; Zhai, Y. Electrospun nanofibers as a wound dressing for treating diabetic foot ulcer. Asian J. Pharm. Sci. 2019, 14, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Homaeigohar, S.; Boccaccini, A.R. Antibacterial biohybrid nanofibers for wound dressings. Acta Biomater. 2020, 107, 25–49. [Google Scholar] [CrossRef] [PubMed]
- Maslakci, N.N.; Ulusoy, S.; Uygun, E.; Çevikbaş, H.; Oksuz, L.; Can, H.K.; Oksuz, A.U. Ibuprofen and acetylsalicylic acid loaded electrospun PVP-dextran nanofiber mats for biomedical applications. Polym. Bull. 2016, 74, 3283–3299. [Google Scholar] [CrossRef]
- Baskakova, A.; Awwad, S.; Jiménez, J.Q.; Gill, H.; Novikov, O.; Khaw, P.T.; Brocchini, S.; Zhilyakova, E.; Williams, G.R. Electrospun formulations of acyclovir, ciprofloxacin and cyanocobalamin for ocular drug delivery. Int. J. Pharm. 2016, 502, 208–218. [Google Scholar] [CrossRef]
- Asawahame, C.; Sutjarittangtham, K.; Eitssayeam, S.; Tragoolpua, Y.; Sirithunyalug, B.; Sirithunyalug, J. Formation of orally fast dissolving fibers containing propolis by electrospinning technique. Chiang Mai J. Sci. 2015, 42, 469–480. [Google Scholar]
- Illangakoon, U.E.; Gill, H.; Shearman, G.C.; Parhizkar, M.; Mahalingam, S.; Chatterton, N.P.; Williams, G.R. Fast dissolving paracetamol/caffeine nanofibers prepared by electrospinning U. Eranka Illangakoon. Int. J. Pharm. 2014, 477, 369–379. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Ma, C.; Wu, Z.; Liang, H.; Yan, P.; Song, J.; Ma, N.; Zhao, Q. Enhanced Bioavailability and Anticancer Effect of Curcumin-Loaded Electrospun Nanofiber: In Vitro and In Vivo Study. Nanoscale Res. Lett. 2015, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Sachdev, A.; Barker, S.A.; Craig, D.Q.M. Design and Characterization of Cyclosporine A-Loaded Nanofibers for Enhanced Drug Dissolution. ACS Omega 2020, 5, 1003–1013. [Google Scholar] [CrossRef]
- Kamble, R.N.; Gaikwad, S.; Maske, A.; Patil, S.S. Fabrication of electrospun nanofibres of BCS II drug for enhanced dissolution and permeation across skin. J. Adv. Res. 2016, 7, 483–489. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Li, D.; Li, J.; Li, S.; Chen, Z.; Yu, D.-G.; Liu, Z.; Guo, J.Z. Electrospun Janus zein–PVP nanofibers provide a two-stage controlled release of poorly water-soluble drugs. Mater. Des. 2020, 196, 109075. [Google Scholar] [CrossRef]
- Rramaswamy, R.; Mani, G.; Jang, H.T. Fabrication of Buccal Dissolving Tetrahydro Curcumin Loaded Polyvidone Fiber Mat: Synthesis, Characterization, and In Vitro Evaluations. J. Appl. Pharm. Sci. 2018, 26–31. [Google Scholar] [CrossRef]
- Li, J.; Pan, H.; Ye, Q.; Shi, C.; Zhang, X.; Pan, W.-S. Carvedilol-loaded polyvinylpyrrolidone electrospun nanofiber film for sublingual delivery. J. Drug Deliv. Sci. Technol. 2020, 58. [Google Scholar] [CrossRef]
- Sriyanti, I.; Edikresnha, D.; Rahma, A.; Munir, M.M.; Rachmawati, H.; Khairurrijal, K. Mangosteen pericarp extract embedded in electrospun PVP nanofiber mats: Physicochemical properties and release mechanism of α-mangostin. Int. J. Nanomed. 2018, 13, 4927–4941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moydeen, A.M.; Padusha, M.S.A.; Thamer, B.M.; Ahamed, N.A.; Al-Enizi, A.M.; El-Hamshary, H.; El-Newehy, M. Single-nozzle Core-shell Electrospun Nanofibers of PVP/Dextran as Drug Delivery System. Fibers Polym. 2019, 20, 2078–2089. [Google Scholar] [CrossRef]
- Bolouri, A. Preparation and characterization of antibacterial nanofibrous trimethoprim/polyvinylpyrrolidone mats as an oral fast-dissolving drug delivery system. Bulgarian Chem. Commun. 2016, 48, 5–14. [Google Scholar]
- El-Newehy, M.H.; Al-Deyab, S.S.; Kenawy, E.-R.; Abdel-Megeed, A. Fabrication of electrospun antimicrobial nanofibers containing metronidazole using nanospider technology. Fibers Polym. 2012, 13, 709–717. [Google Scholar] [CrossRef]











| Bacterial Strain | Zones of Inhibition (mm) | ||
|---|---|---|---|
| Halicin Disc | Blank Fibers | Drug-Loaded Fibers | |
| E. coli (ATCC 22925) | 20 ± 0 | 0 | 27 ± 3 |
| S. aureus (ATCC 29213) | 28 ± 1 | 0 | 21 ± 1 |
| S. aureus (ATCC BAA 977) | 29 ± 1 | 0 | 30 ± 2 |
| A. baumannii (BAA 747) | 25 ± 1 | 0 | 21 ± 2 |
| A. baumannii (MDR 3086) | 22 ± 1 | 0 | 18 ± 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aburayan, W.S.; Booq, R.Y.; BinSaleh, N.S.; Alfassam, H.A.; Bakr, A.A.; Bukhary, H.A.; Alyamani, E.J.; Tawfik, E.A. The Delivery of the Novel Drug ‘Halicin’ Using Electrospun Fibers for the Treatment of Pressure Ulcer against Pathogenic Bacteria. Pharmaceutics 2020, 12, 1189. https://doi.org/10.3390/pharmaceutics12121189
Aburayan WS, Booq RY, BinSaleh NS, Alfassam HA, Bakr AA, Bukhary HA, Alyamani EJ, Tawfik EA. The Delivery of the Novel Drug ‘Halicin’ Using Electrospun Fibers for the Treatment of Pressure Ulcer against Pathogenic Bacteria. Pharmaceutics. 2020; 12(12):1189. https://doi.org/10.3390/pharmaceutics12121189
Chicago/Turabian StyleAburayan, Walaa S., Rayan Y. Booq, Nouf S. BinSaleh, Haya A. Alfassam, Abrar A. Bakr, Haitham A. Bukhary, Essam J. Alyamani, and Essam A. Tawfik. 2020. "The Delivery of the Novel Drug ‘Halicin’ Using Electrospun Fibers for the Treatment of Pressure Ulcer against Pathogenic Bacteria" Pharmaceutics 12, no. 12: 1189. https://doi.org/10.3390/pharmaceutics12121189