Self-Assembling Tacrolimus Nanomicelles for Retinal Drug Delivery
Abstract
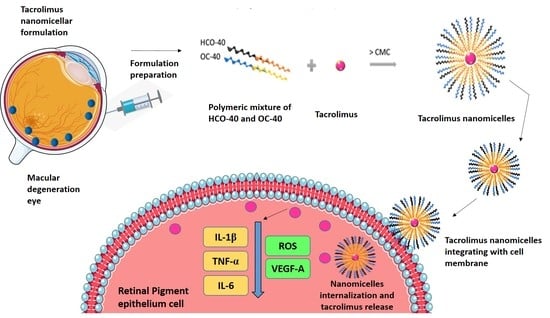
:1. Introduction
2. Materials and Methods
2.1. Design of Experiment for Nanomicelles Preparation
2.2. Preparation of Tacrolimus Nanomicellar Formulation
2.2.1. Formulation Characterization
2.2.2. Drug-Entrapment and Drug-Loading Efficiencies
2.3. Critical Micellar Concentration and Nanomicellar Viscosity Analysis
2.4. Nanomicellar Dilution Study
2.5. 1H NMR Characterization
2.6. In-Vitro Tacrolimus Dissolution and Drug Release
2.7. In-Vitro Cell Viability and Cytotoxicity Assay
2.7.1. MTT Assay
2.7.2. Annexin V/FITC and PI-Staining
2.8. In-Vitro Cellular Uptake Assay
2.8.1. FITC Labelling
2.8.2. In-Vitro Uptake Determination by FACS
2.8.3. In-Vitro Uptake Determination by Confocal Microscopy
2.9. In-Vitro Cellular Transport Determination
2.10. In-Vitro Biocompatibility Assay
2.10.1. Pro-Inflammatory Cytokines in Macrophage Cells
2.10.2. Apoptosis Assay in Kidney Cells
2.11. Evaluation of Reactive Oxygen Species by DCFDA Assay
2.12. In-Vitro Evaluation of TAC-NMF Bioactivity Using ELISA
2.13. Statistical Analysis
3. Results
3.1. Design of Experiment and Formulation Optimization
3.2. Formulation Characterization
3.2.1. Design of Experiment, Size, PDI and Zeta Potential
3.2.2. Entrapment and Loading Efficiencies
3.3. Critical Micellar Concentration and Nanomicellar Viscosity Analysis
3.4. Nanomicellar Dilution Study
3.5. 1H NMR Characterization
3.6. In-Vitro Dissolution and Drug Release
3.7. In-Vitro Viability and Cytotoxicity Assay
3.7.1. MTT Assay
3.7.2. Annexin V/FITC and PI-Staining
3.8. In-Vitro Cellular Uptake and Bio-Distribution of TAC-NMF
3.8.1. FACS Analysis
3.8.2. In-Vitro Bio-Distribution of TAC-NMF Using Confocal Laser Scanning Microscopy Analysis
3.9. In-Vitro Cellular Transport of TAC-NMF
3.10. In-Vitro Biocompatibility Assay
3.10.1. Pro-Inflammatory Cytokines in Macrophage Cells
3.10.2. Apoptosis Assay in Kidney Cells
3.11. Evaluation of Reactive Oxygen Species by DCFDA Assay
3.12. In-Vitro Evaluation of TAC-NMF Bioactivity Using ELISA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| TAC-NMF | tacrolimus nanomicellar formulation |
| TAC | tacrolimus |
| ROS | reactive oxygen species |
| RPE | retinal pigment epithelial cells |
| D407 | human retinal pigment epithelium |
| CCL20.2 | human conjunctival cells |
| RF/6A | human retinal endothelial cells |
| FACS | Fluorescence Assisted Cell Sorting |
| CLSM | confocal laser scanning microscopy |
| IL-1β | Interleukin 1-β |
| IL-6 | Interleukin-6 |
| TNF-α | Tumor Necrosis Factor-α |
| VEGF-A | Vascular endothelial growth factor-A |
| SI | sodium iodate |
References
- Shah, A. Prevalence of Diabetic Retinopathy in the United States, 2011–2014. Value Health 2016, 19, PA199. Available online: https://www.valueinhealthjournal.com/article/S1098-3015(16)01391-7/fulltext (accessed on 5 October 2020). [CrossRef]
- Rudnicka, A.R.; Kapetanakis, V.V.; Jarrar, Z.; Wathern, A.K.; Wormald, R.; Fletcher, A.E.; Cook, D.G.; Owen, C.G. Incidence of Late-Stage Age-Related Macular Degeneration in American Whites: Systematic Review and Meta-analysis. Am. J. Ophthalmol. 2015, 160, 85–93. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Saaddine, J.B.; Chou, C.F.; Cotch, M.F.; Cheng, Y.J.; Geiss, L.S.; Gregg, E.W.; Albright, A.L.; Klein, B.E.; Klein, R. Prevalence of diabetic retinopathy in the United States, 2005–2008. JAMA 2010, 304, 49–56. [Google Scholar] [CrossRef]
- Akyol, E.; Lotery, A. Gene, Cell and Antibody-Based Therapies for the Treatment of Age-Related Macular Degeneration. Biologics 2020, 14, 83–94. [Google Scholar] [CrossRef]
- National Eye Institute. The Most Common Eye Diseases. Available online: https://nei.nih.gov/eyedata (accessed on 5 October 2020).
- Yazdi, M.H.; Faramarzi, M.A.; Nikfar, S.; Falavarjani, K.G.; Abdollahi, M. Ranibizumab and aflibercept for the treatment of wet age-related macular degeneration. Expert Opin. Biol. Ther. 2015, 15, 49–58. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, Y.; Wang, J.X.; Wang, L.F.; Huang, W.R.; Tang, X. Intravitreal ranibizumab injection at the end of vitrectomy for diabetic vitreous hemorrhage (Observational Study). Medicine 2019, 98, e15735. [Google Scholar] [CrossRef]
- Mandal, A.; Pal, D.; Agrahari, V.; Trinh, H.M.; Joseph, M.; Mitra, A.K. Ocular delivery of proteins and peptides: Challenges and novel formulation approaches. Adv. Drug Delivery Rev. 2018, 126, 67–95. [Google Scholar] [CrossRef]
- Datta, S.; Cano, M.; Ebrahimi, K.; Wang, L.; Handa, J.T. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Prog Retin. Eye Res. 2017, 60, 201–218. [Google Scholar] [CrossRef]
- Ardeljan, C.P.; Ardeljan, D.; Abu-Asab, M.; Chan, C.C. Inflammation and Cell Death in Age-Related Macular Degeneration: An Immunopathological and Ultrastructural Model. J. Clin. Med. 2014, 3, 1542–1560. [Google Scholar] [CrossRef] [Green Version]
- Klein, R.; Myers, C.E.; Cruickshanks, K.J.; Gangnon, R.E.; Danforth, L.G.; Sivakumaran, T.A.; Iyengar, S.K.; Tsai, M.Y.; Klein, B.E. Markers of inflammation, oxidative stress, and endothelial dysfunction and the 20-year cumulative incidence of early age-related macular degeneration: The Beaver Dam Eye Study. JAMA Ophthalmol. 2014, 132, 446–455. [Google Scholar] [CrossRef] [Green Version]
- Qiu, A.W.; Bian, Z.; Mao, P.A.; Liu, Q.H. IL-17A exacerbates diabetic retinopathy by impairing Müller cell function via Act1 signaling. Exp. Mol. Med. 2016, 16, 48–58. [Google Scholar] [CrossRef] [Green Version]
- Rubsam, A.; Parikh, S.; Fort, P.E. Role of Inflammation in Diabetic Retinopathy. Int. J. Mol. Sci. 2018, 19, 942. [Google Scholar] [CrossRef] [Green Version]
- Kovacs, K.; Vaczy, A.; Fekete, K.; Kovari, P.; Atlasz, T.; Reglodi, D.; Gabriel, R.; Gallyas, F.; Sumegi, B. PARP Inhibitor Protects Against Chronic Hypoxia/Reoxygenation-Induced Retinal Injury by Regulation of MAPKs, HIF1α, Nrf2, and NFκB. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1478–1490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knickelbein, J.E.; Chan, C.C.; Sen, H.N.; Ferris, F.L.; Nussenblatt, R.B. Inflammatory Mechanisms of Age-related Macular Degeneration. Int. Ophthalmol. Clin. 2015, 55, 63–78. [Google Scholar] [CrossRef] [Green Version]
- Bakri, S.J.; Thorne, J.E.; Ho, A.C.; Ehlers, J.P.; Schoenberger, S.D.; Yeh, S.; Kim, S.J. Safety and Efficacy of Anti-Vascular Endothelial Growth Factor Therapies for Neovascular Age-Related Macular Degeneration: A Report by the American Academy of Ophthalmology. Ophthalmology 2019, 126, 55–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kauppinen, A.; Paterno, J.J.; Blasiak, J.; Salminen, A.; Kaarniranta, K. Inflammation and its role in age-related macular degeneration. Cell. Mol. Life Sci. 2016, 73, 1765–1786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolos, J.M.; Voll, A.M.; Bauder, M.; Hausch, F. FKBP Ligands—Where We Are and Where to Go? Front. Pharmacol. 2018, 9, 1425. [Google Scholar] [CrossRef] [Green Version]
- Cury Martins, J.; Martins, C.; Aoki, V.; Gois, A.F.; Ishii, H.A.; da Silva, E.M. Topical tacrolimus for atopic dermatitis. Cochrane Database Syst. Rev. 2015, 7. [Google Scholar] [CrossRef]
- Baumgart, D.C.; Pintoffl, J.P.; Sturm, A.; Wiedenmann, B.; Dignass, A.U. Tacrolimus is safe and effective in patients with severe steroid-refractory or steroid-dependent inflammatory bowel disease—A long-term follow-up. Am. J. Gastroenterol. 2006, 101, 1048–1056. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Database. Tacrolimus, CID=445643. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Tacrolimus (accessed on 11 September 2019).
- Du, S.; Hiramatsu, N.; Hayakawa, K.; Kasai, A.; Okamura, M.; Huang, T.; Yao, J.; Takeda, M.; Araki, I.; Sawada, N.; et al. Suppression of NF-kappaB by cyclosporin a and tacrolimus (FK506) via induction of the C/EBP family: Implication for unfolded protein response. J. Immunol. 2009, 182, 7201–7211. [Google Scholar] [CrossRef] [Green Version]
- Nakamura-Yanagidaira, T.; Takahashi, Y.; Sano, K.; Murata, T.; Hayashi, T. Development of spontaneous neuropathy in NF-κBp50-deficient mice by calcineurin-signal involving impaired NF-κB activation. Mol. Vis. 2011, 17, 2157–2170. [Google Scholar] [PubMed]
- Yu, Y.; Zhong, J.; Peng, L.; Wang, B.; Li, S.; Huang, H.; Deng, Y.; Zhang, H.; Yang, R.; Wang, C.; et al. Tacrolimus downregulates inflammation by regulating pro/antiinflammatory responses in LPSinduced keratitis. Mol. Med. Rep. 2017, 16, 5855–5862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.S.; Jeong, J.H.; Lee, E.G.; Choi, Y.; Kim, J.H.; Kim, H.R.; Yoo, W.H. Tacrolimus regulates endoplasmic reticulum stress-mediated osteoclastogenesis and inflammation: In-vitro and collagen-induced arthritis mouse model. Cell Biol. Int. 2018, 42, 393–402. [Google Scholar] [CrossRef]
- Su, L.; Ji, J.; Bian, J.; Fu, Y.; Ge, Y.; Yuan, Z. Tacrolimus (FK506) prevents early retinal neovascularization in streptozotocin-induced diabetic mice. Int. Immunopharmacol. 2012, 14, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Gote, V.; Ansong, M.; Pal, D. Prodrugs and nanomicelles to overcome ocular barriers for drug penetration. Expert Opin. Drug Metab. Toxicol. 2020, 16, 885–906. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Gote, V.; Pal, D.; Ogundele, A.; Mitra, A.K. Ocular Pharmacokinetics of a Topical Ophthalmic Nanomicellar Solution of Cyclosporine (Cequa(R)) for Dry Eye Disease. Pharm. Res. 2019, 36, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Cholkar, K.; Khurana, V.; Shah, A.; Agrahari, V.; Bisht, R.; Pal, D.; Mitra, A.K. Topical Formulation of Self-Assembled Antiviral Prodrug Nanomicelles for Targeted Retinal Delivery. Mol. Pharm. 2017, 14, 2056–2069. [Google Scholar] [CrossRef]
- Trinh, H.M.; Cholkar, K.; Joseph, M.; Yang, X.; Mitra, A.K. Clear, Aqueous Topical Drop of Triamcinolone Acetonide. AAPS Pharm. Sci. Tech. 2017, 18, 2466–2478. [Google Scholar] [CrossRef]
- Cholkar, K.; Gilger, B.C.; Mitra, A.K. Topical, Aqueous, Clear Cyclosporine Formulation Design for Anterior and Posterior Ocular Delivery. Transl. Vis. Sci. Technol. 2015, 4. [Google Scholar] [CrossRef] [Green Version]
- Gote, V.; Sikder, S.; Sicotte, J.; Pal, D. Ocular Drug Delivery: Present Innovations and Future Challenges. J. Pharmacol. Exp. Ther. 2019, 370, 602–624. [Google Scholar] [CrossRef]
- Bolla, P.K.; Gote, V.; Singh, M.; Yellepeddi, V.K.; Patel, M.; Pal, D.; Gong, X.; Sambalingam, D.; Renukuntla, J. Preparation and characterization of lutein loaded folate conjugated polymeric nanoparticles. J. Microencapsul. 2020, 37, 502–516. [Google Scholar] [CrossRef] [PubMed]
- Bolla, P.K.; Gote, V.; Singh, M.; Patel, M.; Clark, B.A.; Renukuntla, J. Lutein-Loaded, Biotin-Decorated Polymeric Nanoparticles Enhance Lutein Uptake in Retinal Cells. Pharmaceutics 2020, 12, 798. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.L.; Li, L.H.; Weng, Y.C.; Hua, K.F.; Ju, T.C. Eucalyptus essential oils inhibit the lipopolysaccharide-induced inflammatory response in RAW264.7 macrophages through reducing MAPK and NF-κB pathways. BMC Complement. Med. Ther. 2020, 20, 200. [Google Scholar] [CrossRef] [PubMed]
- Actual by Predicted Plot. Available online: https://www.jmp.com/support/help/en/15.2/index.shtml#page/jmp/actual-by-predicted-plot-2.shtml (accessed on 5 October 2020).
- Mandal, A.; Bisht, R.; Rupenthal, I.D.; Mitra, A.K. Polymeric micelles for ocular drug delivery: From structural frameworks to recent preclinical studies. J. Control Release 2017, 248, 96–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moisseiev, E.; Loewenstein, A. Drug Delivery to the Posterior Segment of the Eye. Dev. Ophthalmol. 2017, 58, 87–101. [Google Scholar] [PubMed]
- Lu, Y.; Yue, Z.; Xie, J.; Wang, W.; Zhu, H.; Zhang, E.; Cao, Z. Micelles with ultralow critical micelle concentration as carriers for drug delivery. Nat. Biomed. Eng. 2018, 2, 318–325. [Google Scholar] [CrossRef]
- Shen, J.; Lu, G.W.; Hughes, P. Targeted Ocular Drug Delivery with Pharmacokinetic/Pharmacodynamic Considerations. Pharm. Res. 2018, 35, 217. [Google Scholar] [CrossRef]
- Cholkar, K.; Gunda, S.; Earla, R.; Pal, D.; Mitra, A.K. Nanomicellar Topical Aqueous Drop Formulation of Rapamycin for Back-of-the-Eye Delivery. AAPS Pharm. Sci. Tech. 2015, 16, 610–622. [Google Scholar] [CrossRef] [Green Version]
- Nayak, K.; Misra, M. Triamcinolone Acetonide-Loaded PEGylated Microemulsion for the Posterior Segment of Eye. ACS Omega. 2020, 5, 7928–7939. [Google Scholar] [CrossRef]
- Grimaudo, M.A.; Pescina, S.; Padula, C.; Santi, P.; Concheiro, A.; Alvarez-Lorenzo, C.; Nicoli, S. Topical application of polymeric nanomicelles in ophthalmology: A review on research efforts for the noninvasive delivery of ocular therapeutics. Expert Opin. Drug Deliv. 2019, 16, 397–413. [Google Scholar] [CrossRef]
- Alshamrani, M.; Sikder, S.; Coulibaly, F.; Mandal, A.; Pal, D.; Mitra, A.K. Self-Assembling Topical Nanomicellar Formulation to Improve Curcumin Absorption Across Ocular Tissues. AAPS Pharm. Sci. Tech. 2019, 20, 254. [Google Scholar] [CrossRef] [PubMed]
- Rozman Peterka, T.; Grahek, R.; Hren, J.; Bastarda, A.; Bergles, J.; Urleb, U. Solid state compatibility study and characterization of a novel degradation product of tacrolimus in formulation. J. Pharm. Biomed. Anal. 2015, 110, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Abouelmagd, S.A.; Sun, B.; Chang, A.C.; Ku, Y.J.; Yeo, Y. Release kinetics study of poorly water-soluble drugs from nanoparticles: Are we doing it right? Mol. Pharm. 2015, 12, 997–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hudson, N.; Celkova, L.; Hopkins, A.; Greene, C.; Storti, F.; Ozaki, E.; Fahey, E.; Theodoropoulou, S.; Kenna, P.F.; Humphries, M.M.; et al. Dysregulated claudin-5 cycling in the inner retina causes retinal pigment epithelial cell atrophy. JCI Insight 2019, 4, 15. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Wang, S.B.; Singh, D.; Zhao, P.Y.; Davis, K.; Chen, B.; Adelman, R.A.; Rizzolo, L.J. Claudin-3 and claudin-19 partially restore native phenotype to ARPE-19 cells via effects on tight junctions and gene expression. Exp. Eye Res. 2016, 151, 179–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koller, A.; Bruckner, D.; Aigner, L.; Reitsamer, H.; Trost, A. Cysteinyl leukotriene receptor 1 modulates autophagic activity in retinal pigment epithelial cells. Sci. Rep. 2020, 10, 17659. [Google Scholar] [CrossRef]
- Naylor, A.; Hopkins, A.; Hudson, N.; Campbell, M. Tight Junctions of the Outer Blood Retina Barrier. Int. J. Mol. Sci. 2019, 21, 211. [Google Scholar] [CrossRef] [Green Version]
- Mazet, R.; Yaméogo, J.B.G.; Wouessidjewe, D.; Choisnard, L.; Gèze, A. Recent Advances in the Design of Topical Ophthalmic Delivery Systems in the Treatment of Ocular Surface Inflammation and Their Biopharmaceutical Evaluation. Pharmaceutics 2020, 12, 570. [Google Scholar] [CrossRef]
- Vaishya, R.D.; Khurana, V.; Patel, S.; Mitra, A.K. Controlled ocular drug delivery with nanomicelles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2014, 6, 422–437. [Google Scholar] [CrossRef] [Green Version]
- Bachu, R.D.; Chowdhury, P.; Al-Saedi, Z.H.F.; Karla, P.K.; Boddu, S.H.S. Ocular Drug Delivery Barriers-Role of Nanocarriers in the Treatment of Anterior Segment Ocular Diseases. Pharmaceutics 2018, 10, 28. [Google Scholar] [CrossRef] [Green Version]
- Qin, S.; Lu, Y.; Rodrigues, G.A. Resveratrol protects RPE cells from sodium iodate by modulating PPARalpha and PPARdelta. Exp. Eye Res. 2014, 118, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Ng, T.K.; Brelén, M.E.; Wu, D.; Wang, J.X.; Chan, K.P.; Yung, J.S.; Cao, D.; Wang, Y.; Zhang, S.; et al. Continuous exposure to non-lethal doses of sodium iodate induces retinal pigment epithelial cell dysfunction. Sci. Rep. 2016, 6, 37279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Li, R.; Xie, J.; Hu, J.; Huang, X.; Ren, F.; Li, L. Protective Effect of Hydrogen on Sodium Iodate-Induced Age-Related Macular Degeneration in Mice. Front. Aging Neurosci. 2018, 10, 389. [Google Scholar] [CrossRef] [PubMed]
- Hanus, J.; Anderson, C.; Sarraf, D.; Ma, J.; Wang, S. Retinal pigment epithelial cell necroptosis in response to sodium iodate. Cell Death Discov. 2016, 2, 16054. [Google Scholar] [CrossRef]
- Ahn, S.M.; Ahn, J.; Cha, S.; Yun, C.; Park, T.K.; Kim, Y.J.; Goo, Y.S.; Kim, S.W. Morphologic and electrophysiologic findings of retinal degeneration after intravitreal sodium iodate injection following vitrectomy in canines. Sci. Rep. 2020, 10, 3588. [Google Scholar] [CrossRef]
- Yang, Y.; Ng, T.K.; Ye, C.; Yip, Y.W.; Law, K.; Chan, S.O.; Pang, C.P. Assessing sodium iodate-induced outer retinal changes in rats using confocal scanning laser ophthalmoscopy and optical coherence tomography. Invest. Ophthalmol. Vis. Sci. 2014, 55, 696–705. [Google Scholar] [CrossRef] [Green Version]
- Kimsa, M.; Strzalka-Mrozik, B.; Kimsa-Dudek, M.; Kruszniewska-Rajs, C.; Gola, J.; Adamska, J.; Mazurek, U. Transforming growth factor β-related genes in human retinal pigment epithelial cells after tacrolimus treatment. Pharmacol. Rep. 2016, 68, 969–974. [Google Scholar] [CrossRef]
- Gote, V.; Pal, D. Poster Presentation. Category: Formulation and Quality.T1030-02-12—Tacrolimus Nanomicellar Eye Drops as a Topical Treatment for Diabetic Retinopathy. AAPS Pharm. Sci. 360 2019. Available online: https://www.eventscribe.com/2019/PharmSci360/fsPopup.asp?efp=SUlFUEhHSFQ4MDkx&PosterID=234587&rnd=0.491479&mode=posterinfo) (accessed on 18 October 2020).
















| Formulation | Coded Design | Uncoded Design | ||||
|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X1 = Sonication Time (Minutes) | X2 = HCO-40 (wt %) | X3 = OC-40 (wt %) | |
| F1 | + | + | + | 25 | 3.5 | 3 |
| F2 | − | + | + | 20 | 3.5 | 3 |
| F3 | 0 | 0 | 0 | 20 | 2 | 2 |
| F4 | + | − | − | 25 | 0.5 | 1 |
| F5 | + | − | + | 25 | 0.5 | 3 |
| F6 | − | + | − | 20 | 3.5 | 1 |
| F7 | 0 | 0 | 0 | 22.5 | 2 | 2 |
| F8 | − | − | + | 20 | 0.5 | 3 |
| F9 | − | − | − | 20 | 0.5 | 1 |
| F10 | + | + | − | 25 | 3.5 | 1 |
| F11 | 0 | 0 | 0 | 22.5 | 2 | 2 |
| Formulation | Pattern | Sonication (min) | HCO-40 wt % | OC-40 wt % | Size (nm) | PDI | Zeta (mV) | %EE | %LE |
|---|---|---|---|---|---|---|---|---|---|
| F1 | +++ | 25 | 3.5 | 3 | 17.22 | 0.29 | 0.658 | 94.74 | 0.59 |
| F2 | −++ | 20 | 3.5 | 3 | 14.59 | 0.2 | 0.837 | 92.49 | 0.55 |
| F3 | 000 | 20 | 2 | 2 | 22.15 | 0.37 | 0.716 | 86.05 | 0.85 |
| F4 | +−− | 25 | 0.5 | 1 | 16.3 | 0.37 | 1.292 | 77.22 | 2.25 |
| F5 | +−+ | 25 | 0.5 | 3 | 16.17 | 0.28 | 1.518 | 77.67 | 1.15 |
| F6 | −+− | 20 | 3.5 | 1 | 15.41 | 0.25 | 0.512 | 97.13 | 0.72 |
| F7 | 000 | 22.5 | 2 | 2 | 22.19 | 0.38 | 0.684 | 89.72 | 0.89 |
| F8 | −−+ | 20 | 0.5 | 3 | 16.35 | 0.32 | 1.081 | 73.53 | 10.9 |
| F9 | −−− | 20 | 0.5 | 1 | 13.34 | 0.26 | 1.896 | 72.71 | 2.12 |
| F10 | ++− | 25 | 3.5 | 1 | 15.57 | 0.16 | 0.611 | 91.17 | 0.68 |
| F11 | 000 | 22.5 | 2 | 2 | 22.72 | 0.23 | 0.819 | 95.58 | 0.95 |
| Dilution Factor | Hydrodynamic Size (nm) | Polydispersity Index (PDI) | Zeta Potential (mV) |
|---|---|---|---|
| 0 | 15.36 | 0.183 | 0.522 |
| 10 | 15.78 | 0.251 | 0.651 |
| 20 | 16.09 | 0.270 | 0.72 |
| 40 | 16.32 | 0.313 | 0.86 |
| 50 | 16.46 | 0.259 | 1.016 |
| 100 | 16.72 | 0.322 | 1.13 |
| 150 | 16.85 | 0.468 | 1.523 |
| 200 | 17.01 | 0.672 | 1.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gote, V.; Mandal, A.; Alshamrani, M.; Pal, D. Self-Assembling Tacrolimus Nanomicelles for Retinal Drug Delivery. Pharmaceutics 2020, 12, 1072. https://doi.org/10.3390/pharmaceutics12111072
Gote V, Mandal A, Alshamrani M, Pal D. Self-Assembling Tacrolimus Nanomicelles for Retinal Drug Delivery. Pharmaceutics. 2020; 12(11):1072. https://doi.org/10.3390/pharmaceutics12111072
Chicago/Turabian StyleGote, Vrinda, Abhirup Mandal, Meshal Alshamrani, and Dhananjay Pal. 2020. "Self-Assembling Tacrolimus Nanomicelles for Retinal Drug Delivery" Pharmaceutics 12, no. 11: 1072. https://doi.org/10.3390/pharmaceutics12111072