Cutaneous Biodistribution: A High-Resolution Methodology to Assess Bioequivalence in Topical Skin Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analytical Methods
2.3. Evaluation of ECZ Skin Delivery In Vitro
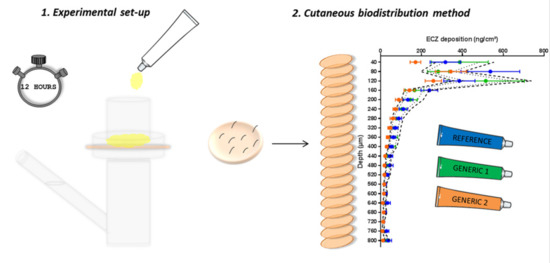
2.3.1. Skin Preparation
2.3.2. ECZ Delivery Under Finite Dose Conditions
2.3.3. Investigation of ECZ Biodistribution Profile
2.4. Data Analysis
2.4.1. Biodistribution Profile
2.4.2. Statistical Analysis
2.4.3. Bioequivalence Assessment by Ratio of Means
3. Results and Discussion
3.1. Biodistribution and Evaluation of Bioequivalence of ECZ Products in Porcine Skin
3.1.1. Validation of the Methodology
3.1.2. Width of the Acceptance Criteria
3.1.3. Comparison of the ECZ Biodistribution from Two Generic Drug Products and the RMP
3.2. Biodistribution and Evaluation of Bioequivalence of ECZ Products in Human Skin
3.3. General Evaluation of the Methodology
3.3.1. Comparison with the Other Accepted/Promising Methods
3.3.2. Statistical Analysis and Acceptance Criteria
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FDA. Guidance for Industry, Bioavailability and Bioequivalence Studies for Orally Administered Drug Products—General Considerations. Available online: https://www.fda.gov/files/drugs/published/Guidance-for-Industry-Bioavailability-and-Bioequivalence-Studies-for-Orally-Administered-Drug-Products---General-Considerations.PDF (accessed on 14 March 2018).
- EMA. Concept Paper on the Development of a Guideline on Quality and Equivalence of Topical Products. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/04/WC500186088.pdf (accessed on 14 March 2018).
- EMA. Draft Guideline on Quality and Equivalence of Topical Products. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guideline-quality-equivalence-topical-products_en.pdf (accessed on 28 March 2019).
- FDA. Guidance for Industry, Topical Dermatologic Corticosteroids: In Vivo Bioequivalence. Available online: https://www.fda.gov/media/70931/download (accessed on 14 March 2018).
- Lapteva, M.; Mondon, K.; Moller, M.; Gurny, R.; Kalia, Y.N. Polymeric micelle nanocarriers for the cutaneous delivery of tacrolimus: A targeted approach for the treatment of psoriasis. Mol. Pharm. 2014, 11, 2989–3001. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zahui, T.; Alberti, I.; Kalia, Y.N. Cutaneous biodistribution of ionizable, biolabile aciclovir prodrugs after short duration topical iontophoresis: Targeted intraepidermal drug delivery. Eur. J. Pharm. Biopharm. 2016, 99, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Lapteva, M.; Mignot, M.; Mondon, K.; Moller, M.; Gurny, R.; Kalia, Y.N. Self-assembled mPEG-hexPLA polymeric nanocarriers for the targeted cutaneous delivery of imiquimod. Eur. J. Pharm. Biopharm. 2019. [Google Scholar] [CrossRef] [PubMed]
- Kandekar, S.G.; Singhal, M.; Sonaje, K.B.; Kalia, Y.N. Polymeric micelle nanocarriers for targeted epidermal delivery of the hedgehog pathway inhibitor vismodegib: Formulation development and cutaneous biodistribution in human skin. Expert. Opin. Drug Deliv. 2019. [Google Scholar] [CrossRef] [PubMed]
- OECD. Guidance Document for the Conduct of Skin Absorption Studies. Available online: https://www.oecd-ilibrary.org/docserver/9789264078796-en.pdf?expires=1554124290&id=id&accname=guest&checksum=CA29912A7F5982B46C4F21EE93B66034 (accessed on 1 April 2019).
- GraphPad: QuickCalcs. Available online: https://www.graphpad.com/quickcalcs/ErrorProp1.cfm (accessed on 28 August 2019).
- Williams, A.C.; Barry, B.W. Penetration enhancers. Adv. Drug Deliv. Rev. 2004, 56, 603–618. [Google Scholar] [CrossRef] [PubMed]
- EMA. Guideline on the Investigation of Bioequivalence. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-investigation-bioequivalence-rev1_en.pdf (accessed on 28 March 2019).
- Vidal. Available online: https://www.vidal.fr/ (accessed on 19 June 2019).
- Walters, K.A. Dermatological and Transdermal Formulations; CRC Press: Boca Raton, FL, USA, 2002; pp. 436–438. [Google Scholar]
- Braddy, A.C.; Davit, B.M.; Stier, E.M.; Conner, D.P. Survey of international regulatory bioequivalence recommendations for approval of generic topical dermatological drug products. AAPS J. 2015, 17, 121–133. [Google Scholar] [CrossRef]
- Herkenne, C.; Naik, A.; Kalia, Y.N.; Hadgraft, J.; Guy, R.H. Pig ear skin ex vivo as a model for in vivo dermatopharmacokinetic studies in man. Pharm. Res. 2006, 23, 1850–1856. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, U.; Kaiser, M.; Toll, R.; Mangelsdorf, S.; Audring, H.; Otberg, N.; Sterry, W.; Lademann, J. Porcine ear skin: An in vitro model for human skin. Skin. Res. Technol. 2007, 13, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Barbero, A.M.; Frasch, H.F. Pig and guinea pig skin as surrogates for human in vitro penetration studies: A quantitative review. Toxicol. In Vitro 2009, 23, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.J.; Pezzone, D.; Badylak, S.F. Regional variations in the histology of porcine skin. Tissue Eng. Part C Methods 2015, 21, 373–384. [Google Scholar] [CrossRef] [PubMed]
- de Hoffmann, E.; Stroobant, V. Spectrométrie de Masse: Cours et Exercices Corrigés; Dunod: Paris, France, 2005; pp. 42–43. [Google Scholar]
- Thevis, M.; Thomas, A.; Pop, V.; Schanzer, W. Ultrahigh pressure liquid chromatography-(tandem) mass spectrometry in human sports drug testing: Possibilities and limitations. J. Chromatogr. A 2013, 1292, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Aller, M.; Gurny, R.; Veuthey, J.L.; Guillarme, D. Coupling ultra high-pressure liquid chromatography with mass spectrometry: Constraints and possible applications. J. Chromatogr. A 2013, 1292, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Schmieder, S.; Krishnamurthy, K. Research techniques made simple: Drug delivery techniques, Part 2: Commonly used techniques to assess topical drug bioavailability. J. Investig. Dermatol. 2016, 136, e43–e49. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; Sousa, J.J.; Veiga, F.; Cardoso, C.; Vitorino, C. Bioequivalence of topical generic products. Part 1: Where are we now? Eur. J. Pharm. Sci. 2018, 123, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Xing, H.; Chen, X.; Xian, L.; Jiang, J.; Yang, T.; Ding, P. Advance in bioequivalence assessment of topical dermatological products. AJPS 2016, 11, 700–707. [Google Scholar] [CrossRef] [Green Version]
- Narkar, Y. Bioequivalence for topical products—An update. Pharm. Res. 2010, 27, 2590–2601. [Google Scholar] [CrossRef]

 ) interval of 80.00–125.00% and (
) interval of 80.00–125.00% and (  ) interval of 69.84–143.19%. P-values were calculated using a Student’s t test; statistically significant differences are denoted by asterisks (* p < 0.05; ** p < 0.005; *** p < 0.0005; **** p < 0.00005).
) interval of 69.84–143.19%. P-values were calculated using a Student’s t test; statistically significant differences are denoted by asterisks (* p < 0.05; ** p < 0.005; *** p < 0.0005; **** p < 0.00005).
 ) interval of 80.00–125.00% and (
) interval of 80.00–125.00% and (  ) interval of 69.84–143.19%. P-values were calculated using a Student’s t test; statistically significant differences are denoted by asterisks (* p < 0.05; ** p < 0.005; *** p < 0.0005; **** p < 0.00005).
) interval of 69.84–143.19%. P-values were calculated using a Student’s t test; statistically significant differences are denoted by asterisks (* p < 0.05; ** p < 0.005; *** p < 0.0005; **** p < 0.00005).
 ) interval of 69.84–143.19%. P-values were calculated using Student’s t test; statistically significant differences are denoted by asterisks (* p < 0.05).
) interval of 69.84–143.19%. P-values were calculated using Student’s t test; statistically significant differences are denoted by asterisks (* p < 0.05).
 ) interval of 69.84–143.19%. P-values were calculated using Student’s t test; statistically significant differences are denoted by asterisks (* p < 0.05).
) interval of 69.84–143.19%. P-values were calculated using Student’s t test; statistically significant differences are denoted by asterisks (* p < 0.05).
 ) interval of 69.84–143.19%. p-values were calculated using Student’s t test analysis; statistically significant differences are denoted by asterisks (* p < 0.05; ** p < 0.005).
) interval of 69.84–143.19%. p-values were calculated using Student’s t test analysis; statistically significant differences are denoted by asterisks (* p < 0.05; ** p < 0.005).
 ) interval of 69.84–143.19%. p-values were calculated using Student’s t test analysis; statistically significant differences are denoted by asterisks (* p < 0.05; ** p < 0.005).
) interval of 69.84–143.19%. p-values were calculated using Student’s t test analysis; statistically significant differences are denoted by asterisks (* p < 0.05; ** p < 0.005).
 ) interval of 0.6984–1.4319.
) interval of 0.6984–1.4319.
 ) interval of 0.6984–1.4319.
) interval of 0.6984–1.4319.
| Parameter | Econazole | Miconazole |
|---|---|---|
| Nature of parent ion | [M + H]+ | [M + H]+ |
| Parent ion (m/z) | 381.1 | 417.1 |
| Daughter ion (m/z) | 124.9 | 158.9 |
| Collision energy (V) | 36 | 34 |
| Cone voltage (V) | 36 | 46 |
| Capillary voltage (kV) | 3.10 | 3.10 |
| Capillary temperature (°C) | 350 | 350 |
| Desolvation gas flow (L/h) | 650 | 650 |
| Cone gas flow (L/h) | 3 | 3 |
| Collision gas flow (L) | 0.15 | 0.15 |
| LM resolution 1 | 2.96 | 2.96 |
| HM resolution 1 | 15 | 15 |
| Ion energy 1 (V) | 0.3 | 0.3 |
| LM resolution 2 | 2.91 | 2.91 |
| HM resolution 2 | 15.24 | 15.24 |
| Ion energy 2 (V) | 0.6 | 0.6 |
| Skin Layers | ECZ 1% RMP Cream | ECZ 1% Generic Cream 1 | ECZ 1% Generic Cream 2 |
|---|---|---|---|
| Total skin | 60% | 53% | 64% |
| Epidermis | 68% | 48% | 70% |
| Upper dermis | 48% | 37% | 59% |
| Lower dermis | 61% | 63% | 63% |
| Assessment of Topical Bioequivalence to RMP | ECZ 1% Generic Cream 1 | ECZ 1% Generic Cream 2 |
|---|---|---|
| Based on the high resolution biodistribution profile | Non-equivalent | Non-equivalent |
| Based on the total skin delivery | Equivalent | Non-equivalent |
| Based on delivery to individual skin layers | Equivalent | Non-equivalent |
| Technique | In Vitro/Ex Vivo | In Vivo | Advantages | Limitations |
|---|---|---|---|---|
| IVRT in vitro release testing | X |
|
| |
| IVPT in vitro permeation testing | X |
|
| |
| Tape stripping/dermatopharmacokinetics | X | X |
|
|
| Vasoconstriction assay | X |
|
| |
| Microdialysis/microperfusion * | X |
|
| |
| Spectroscopic techniques * | X | X |
|
|
| Cutaneous biodistribution method | X |
|
|
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quartier, J.; Capony, N.; Lapteva, M.; Kalia, Y.N. Cutaneous Biodistribution: A High-Resolution Methodology to Assess Bioequivalence in Topical Skin Delivery. Pharmaceutics 2019, 11, 484. https://doi.org/10.3390/pharmaceutics11090484
Quartier J, Capony N, Lapteva M, Kalia YN. Cutaneous Biodistribution: A High-Resolution Methodology to Assess Bioequivalence in Topical Skin Delivery. Pharmaceutics. 2019; 11(9):484. https://doi.org/10.3390/pharmaceutics11090484
Chicago/Turabian StyleQuartier, Julie, Ninon Capony, Maria Lapteva, and Yogeshvar N. Kalia. 2019. "Cutaneous Biodistribution: A High-Resolution Methodology to Assess Bioequivalence in Topical Skin Delivery" Pharmaceutics 11, no. 9: 484. https://doi.org/10.3390/pharmaceutics11090484