A Valid Bisphosphonate Modified Calcium Phosphate-Based Gene Delivery System: Increased Stability and Enhanced Transfection Efficiency In Vitro and In Vivo
Abstract
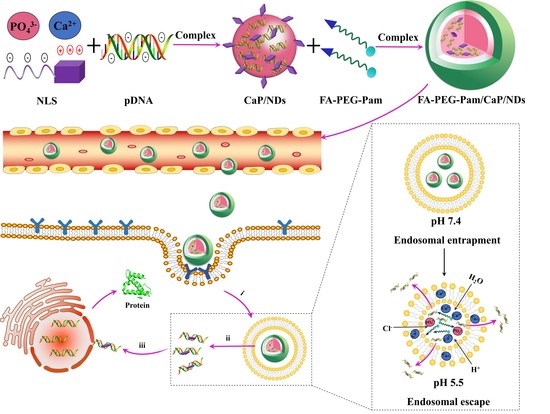
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of FA-PEG-Pam
2.3. Preparation of FA-PEG-Pam/CaP/NDs Nanoparticles
2.4. Characterization of the Nanoparticles
2.5. In Vitro Release of pDNA
2.6. Serum Stability
2.7. In Vitro Cytotoxicity
2.8. Cellular Uptake
2.9. In Vitro Transfection
2.10. Apoptosis Experiment
2.11. Biodistribution in Tumor-Bearing Mice
2.12. Tumor Suppression Efficiency
2.13. Statistics
3. Results and Discussion
3.1. Synthesis and Characteristics of FA-PEG-Pam
3.2. Characterization of FA-PEG-Pam/CaP/NDs Nanoparticles
3.3. Stability Analysis of Nanoparticles
3.4. pH-Sensitive Release of pDNA from FA-PEG-Pam/CaP/NDs Nanoparticles
3.5. MTT Assay
3.6. Cell Uptake Study
3.7. In Vitro Transfection and Expression
3.8. Apoptosis Experiment
3.9. Biodistribution and Tumor Suppression Efficiency of Different Nanoparticles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Foldvari, M.; Chen, D.W.; Nafissi, N.; Calderon, D.; Narsineni, L.; Rafiee, A. Non-viral gene therapy: Gains and challenges of non-invasive administration methods. J. Control. Release 2016, 240, 165–190. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Guo, Y.; Xue, Y.; Niu, W.; Chen, M.; Ma, P.X.; Lei, B. Engineering multifunctional bioactive citric acid-based nanovectors for intrinsical targeted tumor imaging and specific siRNA gene delivery in vitro/in vivo. Biomaterials 2019, 199, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Caffery, B.; Lee, S.J.; Alexander-Bryant, A.A. Vectors for Glioblastoma Gene Therapy: Viral & Non-Viral Delivery Strategies. Nanomaterials 2019, 9, 105. [Google Scholar] [Green Version]
- Bazylińska, U. Rationally designed double emulsion process for co-encapsulation of hybrid cargo in stealth nanocarriers. Colloids Surf. A Physicochem. Eng. Asp. 2017, 532, 476–482. [Google Scholar] [CrossRef]
- Bazylińska, U.; Saczko, J. Nanoemulsion-templated polylelectrolyte multifunctional nanocapsules for DNA entrapment and bioimaging. Colloids Surf. B Biointerfaces 2016, 137, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Oyane, A.; Wang, X.; Sogo, Y.; Ito, A.; Tsurushima, H. Calcium phosphate composite layers for surface-mediated gene transfer. Acta Biomater. 2012, 8, 2034–2046. [Google Scholar] [CrossRef]
- Shen, H.; Tan, J.; Saltzman, W. Surface-mediated gene transfer from nanocomposites of controlled texture. Nat. Mater. 2004, 3, 569–574. [Google Scholar] [CrossRef]
- Graham, F.L.; van der Eb, A.J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology 1973, 52, 456–467. [Google Scholar] [CrossRef]
- Chowdhury, E.H.; Kunou, M.; Nagaoka, M.; Kundu, A.K.; Hoshiba, T.; Akaike, T. High-efficiency gene delivery for expression in mammalian cells by nanoprecipitates of Ca–Mg phosphate. Gene 2004, 341, 77–82. [Google Scholar] [CrossRef]
- Sokolova, V.V.; Radtke, I.; Heumann, R.; Epple, M. Effective transfection of cells with multi-shell calcium phosphate-DNA nanoparticles. Biomaterials 2006, 27, 3147–3153. [Google Scholar] [CrossRef]
- Giger, E.V.; Puigmartí-Luis, J.; Schlatter, R.; Castagner, B.; Dittrich, P.S.; Leroux, J.-C. Gene delivery with bisphosphonate-stabilized calcium phosphate nanoparticles. J. Control. Release 2011, 150, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Fleisch, H. Bisphosphonates: Mechanisms of Action. Adv. Organ Biol. 1998, 5, 835–850. [Google Scholar]
- Russell, R.G.G.; Rogers, M.J. Bisphosphonates: From the laboratory to the clinic and back again. Bone 1999, 25, 97–106. [Google Scholar] [CrossRef]
- Fleisch, H.; Russell, R.G.; Francis, M. Diphosphonates inhibit hydroxyapatite dissolution in vitro and bone resorption in tissue culture in vivo. Am. Assoc. Adv. Sci. 1969, 165, 1261–1264. [Google Scholar] [CrossRef] [PubMed]
- Wolfram, J.; Suri, K.; Yang, Y.; Shen, J.; Celia, C.; Fresta, M.; Zhao, Y.; Shen, H.; Ferrari, M. Shrinkage of pegylated and non-pegylated liposomes in serum. Colloids Surf. B Biointerfaces 2014, 114, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Elechalawar, C.; Sridharan, K.; Pal, A.; Ahmed, M.T.; Yousuf, M.; Adhikari, S.; Banerjee, R. Cationic Folate-Mediated Liposomal Delivery of Bis-Arylidene Oxindole Induces Efficient Melanoma Tumor Regression. Biomater. Sci. 2017, 5, 1898–1909. [Google Scholar] [CrossRef]
- Wu, B.; Yu, P.; Cui, C.; Wu, M.; Zhang, Y.; Liu, L.; Wang, C.-X.; Zhuo, R.-X.; Huang, S.-W. Folate-containing reduction-sensitive lipid–polymer hybrid nanoparticles for targeted delivery of doxorubicin. Biomater. Sci. 2015, 3, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Soniat, M.; Chook, Y.M. Nuclear localization signals for four distinct Karyopherin-β nuclear import systems. Biochem. J. 2015, 468, 353–362. [Google Scholar] [CrossRef]
- Xu, Y.; Liang, W.; Qiu, Y.; Cespi, M.; Palmieri, G.; Mason, A.; Lam, J. Incorporation of a Nuclear Localization Signal in pH Responsive LAH4-L1 Peptide Enhances Transfection and Nuclear Uptake of Plasmid DNA. Mol. Pharm. 2016, 13, 3141–3152. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Jia, L.; Wang, Q.; Hu, H.; Zhao, X.; Chen, D.; Qiao, M. pH/Redox Dual-Responsive Polyplex with Effective Endosomal Escape for Codelivery of siRNA and Doxorubicin against Drug-Resistant Cancer Cells. ACS Appl. Mater. Interfaces 2019, 11, 16296–16310. [Google Scholar] [CrossRef]
- Roy, I.; Mitra, S.; Maitra, A.; Mozumdar, S. Calcium Phosphate Nanoparticles as Novel Non-Viral Vectors for Targeted Gene Delivery. Int. J. Pharm. 2003, 250, 25–33. [Google Scholar] [CrossRef]
- Kakizawa, Y.; Furukawa, S.; Kataoka, K. Block copolymer-coated calcium phosphate nanoparticles sensing intracellular environment for oligodeoxynucleotide and siRNA delivery. J. Control. Release 2004, 97, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Kakizawa, Y.; Furukawa, S.; Ishii, A.; Kataoka, K. Organic–inorganic hybrid-nanocarrier of siRNA constructing through the self-assembly of calcium phosphate and PEG-based block aniomer. J. Control. Release 2006, 111, 368–370. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, M.; Zhang, Y.; Zeng, J.; Omari-Siaw, E.; Yu, J.; Xu, X. In Vitro Release and Bioavailability of Silybin from Micelle-Templated Porous Calcium Phosphate Microparticles. AAps Pharmscitech 2015, 17, 1232–1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, S.; Lee, S.; Kim, H.; Ham, J.; Seo, J.-H.; Mok, Y.; Noh, M.; Lee, Y. Preparation of pH-sensitive CaP nanoparticles coated with a phosphate-based block copolymer for efficient gene delivery. Polymer 2012, 53, 4678–4685. [Google Scholar] [CrossRef]
- Kakizawa, Y.; Kataoka, K. Block Copolymer Self-Assembly into Monodispersive Nanoparticles with Hybrid Core of Antisense DNA and Calcium Phosphate. Langmuir 2002, 18, 4539–4543. [Google Scholar] [CrossRef]
- Li, J.; Chen, Y.-C.; Tseng, Y.-C.; Mozumdar, S.; Huang, L. Biodegradable calcium phosphate nanoparticle with lipid coating for systemic siRNA delivery. J. Control. Release 2010, 142, 416–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Kataoka, K. Nano-structured composites based on calcium phosphate for cellular delivery of therapeutic and diagnostic agents. Nano Today 2009, 4, 508–517. [Google Scholar] [CrossRef]
- Fleisch, H. Bisphosphonates: Mechanisms of Action and Clinical Use. Physiol. Pharmacol. Bone 1993, 107, 377–418. [Google Scholar]
- Alghamdi, H.S.; Bosco, R.; Both, S.K.; Iafisco, M.; Leeuwenburgh, S.C.G.; Jansen, J.A.; van den Beucken, J.J.J.P. Synergistic effects of bisphosphonate and calcium phosphate nanoparticles on peri-implant bone responses in osteoporotic rats. Biomaterials 2014, 35, 5482–5490. [Google Scholar] [CrossRef]
- Lee, D.; Upadhye, K.; Kumta, P.N. Nano-sized calcium phosphate (CaP) carriers for non-viral gene delivery. Mater. Sci. Eng. B 2012, 177, 289–302. [Google Scholar] [CrossRef]
- Varkouhi, A.K.; Scholte, M.; Storm, G.; Haisma, H.J. Endosomal escape pathways for delivery of biologicals. J. Control. Release 2011, 151, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Park, H.; Yu, S.H.; Na, K.; Oh, T.K.; Lee, S.E. Dendritic Cell-Targeted pH-Responsive Extracellular Vesicles for Anticancer Vaccination. Pharmaceutics 2019, 11, 54. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Parekh, S.H.; Xu, P.Z. Enhanced Cellular Delivery and Biocompatibility of a Small Layered Double Hydroxide–Liposome Composite System. Pharmaceutics 2014, 6, 584–598. [Google Scholar] [CrossRef] [PubMed]
- Bisht, S.; Bhakta, G.; Mitra, S.; Maitra, A. pDNA loaded calcium phosphate nanoparticles: Highly efficient non-viral vector for gene delivery. Int. J. Pharm. 2005, 288, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Li, Y.; Xu, R.; Li, S.; Hu, H.; Xiao, C.; Wu, H.; Zhu, L.; Ming, J.; Chu, Z.; et al. Self-assembly of folic acid dextran conjugates for cancer chemotherapy. Nanoscale 2018, 10, 17265–17274. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Sun, Z.; Li, Y.; Guo, Y.; Liao, Y.-H.; Liu, M.; Wang, X. Folate-modified Annonaceous acetogenins nanosuspensions and their improved antitumor efficacy. Int. J. Nanomed. 2017, 12, 5053–5067. [Google Scholar] [CrossRef]
- Shirani, M.P.; Rezaei, B.; Khayamian, T.; Dinari, M.; Shamili, F.H.; Ramezani, M.; Alibolandi, M. Ingenious pH-sensitive etoposide loaded folic acid decorated mesoporous silica-carbon dot with carboxymethyl-βcyclodextrin gatekeeper for targeted drug delivery and imaging. Mater. Sci. Eng. C 2018, 92, 892–901. [Google Scholar] [CrossRef]
- Xu, J.; Xu, B.; Shou, D.; Qin, F.; Xu, Y.; Hu, Y. Characterization and evaluation of a folic acid receptor-targeted cyclodextrin complex as an anticancer drug delivery system. Eur. J. Pharm. Sci. 2016, 83, 132–142. [Google Scholar] [CrossRef]
- Sabharanjak, S.; Mayor, S. Folate receptor endocytosis and trafficking. Adv. Drug Deliv. Rev. 2004, 56, 1099–1109. [Google Scholar] [CrossRef]
- Sun, Y.; Xian, L.; Xing, H.; Yu, J.; Yang, Z.; Yang, T.; Yang, L.; Ding, P. Factors Influencing the Nuclear Targeting Ability of Nuclear Localization Signals. J. Drug Target. 2016, 24, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhao, G.; Zhu, X.; Jiang, K.; Wu, H.; Deng, G.; Qiu, C. Sodium selenite induces apoptosis via ROS-mediated NF-κB signaling and activation of the Bax-caspase-9-caspase-3 axis in 4T1 cells. J. Cell. Physiol. 2018, 234, 2511–2522. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-T.; Lin, C.-L.; Lin, T.-Y.; Cheng, C.-W.; Yang, S.-F.; Lin, C.-L.; Wu, C.-C.; Hsieh, Y.-H.; Tsai, J.-P. Synergistic effect of fisetin combined with sorafenib in human cervical cancer HeLa cells through activation of death receptor-5 mediated caspase-8/caspase-3 and the mitochondria-dependent apoptotic pathway. Tumor Biol. 2016, 37, 6987–6996. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Wanggou, S.; Liu, Q.; Li, X.; Liu, J.; Wu, M. A brain-specific isoform of apoptosis-inducing factor 2 attenuates ischemia-induced oxidative stress in HT22 cells. Neurochem. Int. 2018, 112, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Fan, T.-J. Clonidine Induces Apoptosis of Human Corneal Epithelial Cells through Death Receptors-Mediated, Mitochondria-Dependent Signaling Pathway. Toxicol. Sci. 2017, 156, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Fan, T.-J. Tetracaine induces apoptosis through a mitochondrion-dependent pathway in human corneal stromal cells in vitro. Cutan. Ocul. Toxicol. 2018, 37, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, S.-P.; Cai, Y.-Q. Cytoprotective effects of selenium on cadmium-induced LLC-PK1 cells apoptosis by activating JNK pathway. Toxicol. In Vitro 2007, 21, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Vogelstein, B.; Lane, D.; Levine, A.J. Surfing the p53 network. Nature 2000, 408, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.J.; Hu, W.; Feng, Z. The P53 pathway: What questions remain to be explored? Cell Death Differ. 2006, 13, 1027–1036. [Google Scholar] [CrossRef] [PubMed]









| Particle Size (nm) | PDI | Zeta Potential (mV) | |
|---|---|---|---|
| mPEG-Pam/CaP/NDs | 156.4 ± 10.2 | 0.23 ± 0.05 | −2.33 ± 0.28 |
| FA-PEG-Pam/CaP/NDs | 164.2 ± 7.6 | 0.17 ± 0.04 | −1.23 ± 0.14 |
| FA-PEG-Pam/CaP/pDNA | 166.2 ± 15.2 | 0.15 ± 0.06 | −0.83 ± 0.26 |
| CaP/NDs | 413.5 ± 17.9 | 0.26 ± 0.08 | 2.73 ± 1.13 |
| Particle Size (nm) | PDI | Zeta Potential (mV) | |
|---|---|---|---|
| mPEG-Pam/CaP/NDs | 161.4 ± 8.3 | 0.21 ± 0.06 | −1.47 ± 0.32 |
| FA-PEG-Pam/CaP/NDs | 169.0 ± 9.7 | 0.14 ± 0.05 | −1.76 ± 0.21 |
| FA-PEG-Pam/CaP/pDNA | 164.2 ± 12.6 | 0.20 ± 0.07 | 0.43 ± 0.15 |
| CaP/NDs | 913.5 ± 56.9 | 0.27 ± 0.07 | 3.68 ± 1.45 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Li, J.; Chen, D.; Hu, H. A Valid Bisphosphonate Modified Calcium Phosphate-Based Gene Delivery System: Increased Stability and Enhanced Transfection Efficiency In Vitro and In Vivo. Pharmaceutics 2019, 11, 468. https://doi.org/10.3390/pharmaceutics11090468
Zhao M, Li J, Chen D, Hu H. A Valid Bisphosphonate Modified Calcium Phosphate-Based Gene Delivery System: Increased Stability and Enhanced Transfection Efficiency In Vitro and In Vivo. Pharmaceutics. 2019; 11(9):468. https://doi.org/10.3390/pharmaceutics11090468
Chicago/Turabian StyleZhao, Ming, Ji Li, Dawei Chen, and Haiyang Hu. 2019. "A Valid Bisphosphonate Modified Calcium Phosphate-Based Gene Delivery System: Increased Stability and Enhanced Transfection Efficiency In Vitro and In Vivo" Pharmaceutics 11, no. 9: 468. https://doi.org/10.3390/pharmaceutics11090468