Brain Delivery of a Potent Opioid Receptor Agonist, Biphalin during Ischemic Stroke: Role of Organic Anion Transporting Polypeptide (OATP)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture: iPSCs Differentiation to BMECs
2.2. Measurement of Barrier Function: TEER and Paracellular Permeability
2.3. Measurement of Biphalin: LC-MS/MS Method
2.4. Biphalin Uptake Studies in iPSC-BMECs
2.5. Biphalin Transcellular Transport Studies
2.6. Oxygen Glucose Deprivation (OGD) and Reperfusion
2.7. Immunocytochemistry
2.8. Flow Cytometry
2.9. Data Analysis
3. Results
3.1. Selection of OATP1 Expressing Brain Endothelial Cells
3.2. OATP1 Contributes to Biphalin Uptake and Transport
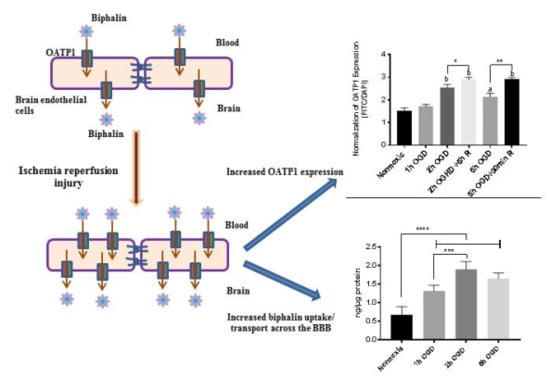
3.3. Effect of OGD-Reperfusion on Biphalin Uptake
3.4. OATP1 Expression Increased During OGD-Reperfusion
3.5. OATP1 Contributes in the Uptake and Transport of Biphalin across the BBB
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef] [PubMed]
- Dong, X. Current Strategies for Brain Drug Delivery. Theranostics 2018, 8, 1481–1493. [Google Scholar] [CrossRef] [PubMed]
- Abdullahi, W.; Davis, T.P.; Ronaldson, P.T. Functional Expression of P-glycoprotein and Organic Anion Transporting Polypeptides at the Blood-Brain Barrier: Understanding Transport Mechanisms for Improved CNS Drug Delivery? AAPS J. 2017, 19, 931–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaidya, B.; Sifat, A.E.; Karamyan, V.T.; Abbruscato, T.J. The neuroprotective role of the brain opioid system in stroke injury. Drug Discov. Today 2018, 23, 1385–1395. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Shah, K.; Wang, H.; Karamyan, V.T.; Abbruscato, T.J. Characterization of neuroprotective effects of biphalin, an opioid receptor agonist, in a model of focal brain ischemia. J. Pharmacol. Exp. Ther. 2011, 339, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, H.; Shah, K.; Karamyan, V.T.; Abbruscato, T.J. Opioid receptor agonists reduce brain edema in stroke. Brain Res. 2011, 1383, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Islam, M.R.; Karamyan, V.T.; Abbruscato, T.J. In vitro and in vivo efficacy of a potent opioid receptor agonist, biphalin, compared to subtype-selective opioid receptor agonists for stroke treatment. Brain Res. 2015, 1609, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.R.; Yang, L.; Lee, Y.S.; Hruby, V.J.; Karamyan, V.T.; Abbruscato, T.J. Enkephalin-Fentanyl Multifunctional Opioids as Potential Neuroprotectants for Ischemic Stroke Treatment. Curr. Pharm. Des. 2016, 22, 6459–6468. [Google Scholar] [CrossRef]
- Abbruscato, T.J.; Thomas, S.A.; Hruby, V.J.; Davis, T.P. Brain and spinal cord distribution of biphalin: Correlation with opioid receptor density and mechanism of CNS entry. J. Neurochem. 1997, 69, 1236–1245. [Google Scholar] [CrossRef]
- Ronaldson, P.T.; Finch, J.D.; Demarco, K.M.; Quigley, C.E.; Davis, T.P. Inflammatory pain signals an increase in functional expression of organic anion transporting polypeptide 1a4 at the blood-brain barrier. J. Pharmacol. Exp. Ther. 2011, 336, 827–839. [Google Scholar] [CrossRef]
- Thompson, B.J.; Sanchez-Covarrubias, L.; Slosky, L.M.; Zhang, Y.; Laracuente, M.L.; Ronaldson, P.T. Hypoxia/reoxygenation stress signals an increase in organic anion transporting polypeptide 1a4 (Oatp1a4) at the blood-brain barrier: relevance to CNS drug delivery. J. Cereb. Blood Flow Metab. 2014, 34, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Cen, J.; Liu, L.; Li, M.S.; He, L.; Wang, L.J.; Liu, Y.Q.; Liu, M.; Ji, B.S. Alteration in P-glycoprotein at the blood-brain barrier in the early period of MCAO in rats. J. Pharm. Pharmacol. 2013, 65, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Dazert, P.; Suofu, Y.; Grube, M.; Popa-Wagner, A.; Kroemer, H.K.; Jedlitschky, G.; Kessler, C. Differential regulation of transport proteins in the periinfarct region following reversible middle cerebral artery occlusion in rats. Neuroscience 2006, 142, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Spudich, A.; Kilic, E.; Xing, H.; Kilic, U.; Rentsch, K.M.; Wunderli-Allenspach, H.; Bassetti, C.L.; Hermann, D.M. Inhibition of multidrug resistance transporter-1 facilitates neuroprotective therapies after focal cerebral ischemia. Nat. Neurosci. 2006, 9, 487–488. [Google Scholar] [CrossRef]
- Sifat, A.E.; Vaidya, B.; Villalba, H.; Albekairi, T.H.; Abbruscato, T.J. Neurovascular unit transport responses to ischemia and common coexisting conditions: Smoking and diabetes. Am. J. Physiol. Cell Physiol. 2019, 316, C2–C15. [Google Scholar] [CrossRef] [PubMed]
- Hettiarachchi, K.; Ridge, S.; Thomas, D.W.; Olson, L.; Obi, C.R.; Singh, D. Characterization and analysis of biphalin: An opioid peptide with a palindromic sequence. J. Pept. Res. 2001, 57, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Lipkowski, A.W.; Konecka, A.M.; Sroczynska, I. Double-enkephalins--synthesis, activity on guinea-pig ileum, and analgesic effect. Peptides 1982, 3, 697–700. [Google Scholar] [CrossRef]
- Reichel, C.; Gao, B.; Van Montfoort, J.; Cattori, V.; Rahner, C.; Hagenbuch, B.; Stieger, B.; Kamisako, T.; Meier, P.J. Localization and function of the organic anion-transporting polypeptide Oatp2 in rat liver. Gastroenterology 1999, 117, 688–695. [Google Scholar] [CrossRef]
- Gao, B.; Hagenbuch, B.; Kullak-Ublick, G.A.; Benke, D.; Aguzzi, A.; Meier, P.J. Organic anion-transporting polypeptides mediate transport of opioid peptides across blood-brain barrier. J. Pharmacol. Exp. Ther. 2000, 294, 73–79. [Google Scholar] [PubMed]
- Ose, A.; Kusuhara, H.; Endo, C.; Tohyama, K.; Miyajima, M.; Kitamura, S.; Sugiyama, Y. Functional characterization of mouse organic anion transporting peptide 1a4 in the uptake and efflux of drugs across the blood-brain barrier. Drug Metab. Dispos. 2010, 38, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Ronaldson, P.T.; Davis, T.P. Targeting transporters: Promoting blood-brain barrier repair in response to oxidative stress injury. Brain Res. 2015, 1623, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Meier-Abt, F.; Mokrab, Y.; Mizuguchi, K. Organic anion transporting polypeptides of the OATP/SLCO superfamily: identification of new members in nonmammalian species, comparative modeling and a potential transport mode. J. Membr. Biol. 2005, 208, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Franke, R.M.; Scherkenbach, L.A.; Sparreboom, A. Pharmacogenetics of the organic anion transporting polypeptide 1A2. Pharmacogenomics 2009, 10, 339–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lippmann, E.S.; Azarin, S.M.; Kay, J.E.; Nessler, R.A.; Wilson, H.K.; Al-Ahmad, A.; Palecek, S.P.; Shusta, E.V. Derivation of blood-brain barrier endothelial cells from human pluripotent stem cells. Nat. Biotechnol. 2012, 30, 783–791. [Google Scholar] [CrossRef]
- Lippmann, E.S.; Al-Ahmad, A.; Azarin, S.M.; Palecek, S.P.; Shusta, E.V. A retinoic acid-enhanced, multicellular human blood-brain barrier model derived from stem cell sources. Sci. Rep. 2014, 4, 4160. [Google Scholar] [CrossRef] [Green Version]
- Al-Ahmad, A.J. Comparative study of expression and activity of glucose transporters between stem cell-derived brain microvascular endothelial cells and hCMEC/D3 cells. Am. J. Physiol. Cell Physiol. 2017, 313, C421–C429. [Google Scholar] [CrossRef]
- Ribecco-Lutkiewicz, M.; Sodja, C.; Haukenfrers, J.; Haqqani, A.S.; Ly, D.; Zachar, P.; Baumann, E.; Ball, M.; Huang, J.; Rukhlova, M.; et al. A novel human induced pluripotent stem cell blood-brain barrier model: Applicability to study antibody-triggered receptor-mediated transcytosis. Sci. Rep. 2018, 8, 1873. [Google Scholar] [CrossRef] [PubMed]
- Qian, T.; Maguire, S.E.; Canfield, S.G.; Bao, X.; Olson, W.R.; Shusta, E.V.; Palecek, S.P. Directed differentiation of human pluripotent stem cells to blood-brain barrier endothelial cells. Sci. Adv. 2017, 3, e1701679. [Google Scholar] [CrossRef] [PubMed]
- Page, S.; Patel, R.; Raut, S.; Al-Ahmad, A. Neurological diseases at the blood-brain barrier: Stemming new scientific paradigms using patient-derived induced pluripotent cells. Biochim. Biophys. Acta Mol. Basis Dis. 2018. [Google Scholar] [CrossRef]
- Weksler, B.; Romero, I.A.; Couraud, P.O. The hCMEC/D3 cell line as a model of the human blood brain barrier. Fluids Barriers CNS 2013, 10, 16. [Google Scholar] [CrossRef]
- Yang, T.; Roder, K.E.; Abbruscato, T.J. Evaluation of bEnd5 cell line as an in vitro model for the blood-brain barrier under normal and hypoxic/aglycemic conditions. J. Pharm. Sci. 2007, 96, 3196–3213. [Google Scholar] [CrossRef] [PubMed]
- Kovalevich, J.; Langford, D. Considerations for the use of SH-SY5Y neuroblastoma cells in neurobiology. Methods Mol. Biol. 2013, 1078, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Page, S.; Munsell, A.; Al-Ahmad, A.J. Cerebral hypoxia/ischemia selectively disrupts tight junctions complexes in stem cell-derived human brain microvascular endothelial cells. Fluids Barriers CNS 2016, 13, 16. [Google Scholar] [CrossRef] [PubMed]
- Vemula, S.; Roder, K.E.; Yang, T.; Bhat, G.J.; Thekkumkara, T.J.; Abbruscato, T.J. A functional role for sodium-dependent glucose transport across the blood-brain barrier during oxygen glucose deprivation. J. Pharmacol. Exp. Ther. 2009, 328, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Hollmann, E.K.; Bailey, A.K.; Potharazu, A.V.; Neely, M.D.; Bowman, A.B.; Lippmann, E.S. Accelerated differentiation of human induced pluripotent stem cells to blood-brain barrier endothelial cells. Fluids Barriers CNS 2017, 14, 9. [Google Scholar] [CrossRef] [PubMed]
- Egleton, R.D.; Abbruscato, T.J.; Thomas, S.A.; Davis, T.P. Transport of opioid peptides into the central nervous system. J. Pharm. Sci. 1998, 87, 1433–1439. [Google Scholar] [CrossRef] [PubMed]
- Villalba, H.; Shah, K.; Albekairi, T.H.; Sifat, A.E.; Vaidya, B.; Abbruscato, T.J. Potential role of myo-inositol to improve ischemic stroke outcome in diabetic mouse. Brain Res. 2018, 1699, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Leake, B.F.; Kim, R.B.; Ho, R.H. Contribution of Organic Anion-Transporting Polypeptides 1A/1B to Doxorubicin Uptake and Clearance. Mol. Pharmacol. 2017, 91, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Borlongan, C.V.; Wang, Y.; Su, T.P. Delta opioid peptide (D-Ala 2, D-Leu 5) enkephalin: Linking hibernation and neuroprotection. Front. Biosci. 2004, 9, 3392–3398. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Xu, H.; Lu, J.; Zhu, Y.; Jiang, H. Neuroprotection by the kappa-opioid receptor agonist, BRL52537, is mediated via up-regulating phosphorylated signal transducer and activator of transcription-3 in cerebral ischemia/reperfusion injury in rats. Neurochem. Res. 2013, 38, 2305–2312. [Google Scholar] [CrossRef] [PubMed]
- Eftekhar-Vaghefi, S.; Esmaeili-Mahani, S.; Elyasi, L.; Abbasnejad, M. Involvement of Mu Opioid Receptor Signaling in the Protective Effect of Opioid against 6-Hydroxydopamine-Induced SH-SY5Y Human Neuroblastoma Cells Apoptosis. Basic Clin. Neurosci. 2015, 6, 171–178. [Google Scholar] [PubMed]
- Shen, K.F.; Crain, S.M. Biphalin, an enkephalin analog with unexpectedly high antinociceptive potency and low dependence liability in vivo, selectively antagonizes excitatory opioid receptor functions of sensory neurons in culture. Brain Res. 1995, 701, 158–166. [Google Scholar] [CrossRef]
- Lesniak, A.; Bochynska-Czyz, M.; Sacharczuk, M.; Benhye, S.; Misicka, A.; Bujalska-Zadrozny, M.; Lipkowski, A.W. Biphalin preferentially recruits peripheral opioid receptors to facilitate analgesia in a mouse model of cancer pain - A comparison with morphine. Eur. J. Pharm. Sci. 2016, 89, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Misicka, A.; Lipkowski, A.W.; Horvath, R.; Davis, P.; Porreca, F.; Yamamura, H.I.; Hruby, V.J. Structure-activity relationship of biphalin. The synthesis and biological activities of new analogues with modifications in positions 3 and 4. Life Sci. 1997, 60, 1263–1269. [Google Scholar] [CrossRef]
- Horan, P.J.; Mattia, A.; Bilsky, E.J.; Weber, S.; Davis, T.P.; Yamamura, H.I.; Malatynska, E.; Appleyard, S.M.; Slaninova, J.; Misicka, A.; et al. Antinociceptive profile of biphalin, a dimeric enkephalin analog. J. Pharmacol. Exp. Ther. 1993, 265, 1446–1454. [Google Scholar] [PubMed]
- Lipkowski, A.W.; Konecka, A.M.; Sroczynska, I.; Przewlocki, R.; Stala, L.; Tam, S.W. Bivalent opioid peptide analogues with reduced distances between pharmacophores. Life Sci. 1987, 40, 2283–2288. [Google Scholar] [CrossRef]
- Sobczak, M.; Pilarczyk, A.; Jonakowski, M.; Jarmuz, A.; Salaga, M.; Lipkowski, A.W.; Fichna, J. Anti-inflammatory and antinociceptive action of the dimeric enkephalin peptide biphalin in the mouse model of colitis: new potential treatment of abdominal pain associated with inflammatory bowel diseases. Peptides 2014, 60, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Feliciani, F.; Pinnen, F.; Stefanucci, A.; Costante, R.; Cacciatore, I.; Lucente, G.; Mollica, A. Structure-activity relationships of biphalin analogs and their biological evaluation on opioid receptors. Mini. Rev. Med. Chem. 2013, 13, 11–33. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, M.; Suzuki, T.; Narita, M.; Lipkowski, A.W. The opioid peptide analogue biphalin induces less physical dependence than morphine. Life Sci. 2001, 69, 1023–1028. [Google Scholar] [CrossRef]
- Badzynska, B.; Lipkowski, A.W.; Sadowski, J. An antihypertensive opioid: Biphalin, a synthetic non-addictive enkephalin analog decreases blood pressure in spontaneously hypertensive rats. Pharmacol. Rep. 2016, 68, 51–55. [Google Scholar] [CrossRef]
- Narita, M.; Kuzumaki, N.; Miyatake, M.; Sato, F.; Wachi, H.; Seyama, Y.; Suzuki, T. Role of delta-opioid receptor function in neurogenesis and neuroprotection. J. Neurochem. 2006, 97, 1494–1505. [Google Scholar] [CrossRef] [PubMed]
- Abbruscato, T.J.; Williams, S.A.; Misicka, A.; Lipkowski, A.W.; Hruby, V.J.; Davis, T.P. Blood-to-central nervous system entry and stability of biphalin, a unique double-enkephalin analog, and its halogenated derivatives. J. Pharmacol. Exp. Ther. 1996, 276, 1049–1057. [Google Scholar] [PubMed]
- Bronger, H.; Konig, J.; Kopplow, K.; Steiner, H.H.; Ahmadi, R.; Herold-Mende, C.; Keppler, D.; Nies, A.T. ABCC drug efflux pumps and organic anion uptake transporters in human gliomas and the blood-tumor barrier. Cancer Res. 2005, 65, 11419–11428. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.W.; Bao, J.Q.; Ke, A.B.; Manro, J.R.; Fallon, J.K.; Smith, P.C.; Zamek-Gliszczynski, M.J. Utility of Oatp1a/1b-knockout and OATP1B1/3-humanized mice in the study of OATP-mediated pharmacokinetics and tissue distribution: case studies with pravastatin, atorvastatin, simvastatin, and carboxydichlorofluorescein. Drug Metab. Dispos. 2014, 42, 182–192. [Google Scholar] [CrossRef] [PubMed]
- van de Steeg, E.; van Esch, A.; Wagenaar, E.; van der Kruijssen, C.M.; van Tellingen, O.; Kenworthy, K.E.; Schinkel, A.H. High impact of Oatp1a/1b transporters on in vivo disposition of the hydrophobic anticancer drug paclitaxel. Clin. Cancer Res. 2011, 17, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Durmus, S.; Naik, J.; Buil, L.; Wagenaar, E.; van Tellingen, O.; Schinkel, A.H. In vivo disposition of doxorubicin is affected by mouse Oatp1a/1b and human OATP1A/1B transporters. Int. J. Cancer 2014, 135, 1700–1710. [Google Scholar] [CrossRef]
- Sifat, A.E.; Vaidya, B.; Abbruscato, T.J. Blood-Brain Barrier Protection as a Therapeutic Strategy for Acute Ischemic Stroke. AAPS J. 2017, 19, 957–972. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Sangha, K.S.; Khatri, P. Drug treatment of acute ischemic stroke. Am. J. Cardiovasc. Drugs 2013, 13, 57–69. [Google Scholar] [CrossRef]
- Chamorro, A.; Dirnagl, U.; Urra, X.; Planas, A.M. Neuroprotection in acute stroke: Targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. 2016, 15, 869–881. [Google Scholar] [CrossRef]
- Banks, W.A. From blood-brain barrier to blood-brain interface: new opportunities for CNS drug delivery. Nat. Rev. Drug Discov. 2016, 15, 275–292. [Google Scholar] [CrossRef]
- Abbott, N.J. Astrocyte-endothelial interactions and blood-brain barrier permeability. J. Anat. 2002, 200, 629–638. [Google Scholar] [CrossRef] [PubMed]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albekairi, T.H.; Vaidya, B.; Patel, R.; Nozohouri, S.; Villalba, H.; Zhang, Y.; Lee, Y.S.; Al-Ahmad, A.; Abbruscato, T.J. Brain Delivery of a Potent Opioid Receptor Agonist, Biphalin during Ischemic Stroke: Role of Organic Anion Transporting Polypeptide (OATP). Pharmaceutics 2019, 11, 467. https://doi.org/10.3390/pharmaceutics11090467
Albekairi TH, Vaidya B, Patel R, Nozohouri S, Villalba H, Zhang Y, Lee YS, Al-Ahmad A, Abbruscato TJ. Brain Delivery of a Potent Opioid Receptor Agonist, Biphalin during Ischemic Stroke: Role of Organic Anion Transporting Polypeptide (OATP). Pharmaceutics. 2019; 11(9):467. https://doi.org/10.3390/pharmaceutics11090467
Chicago/Turabian StyleAlbekairi, Thamer H, Bhuvaneshwar Vaidya, Ronak Patel, Saeideh Nozohouri, Heidi Villalba, Yong Zhang, Yeon Sun Lee, Abraham Al-Ahmad, and Thomas J Abbruscato. 2019. "Brain Delivery of a Potent Opioid Receptor Agonist, Biphalin during Ischemic Stroke: Role of Organic Anion Transporting Polypeptide (OATP)" Pharmaceutics 11, no. 9: 467. https://doi.org/10.3390/pharmaceutics11090467