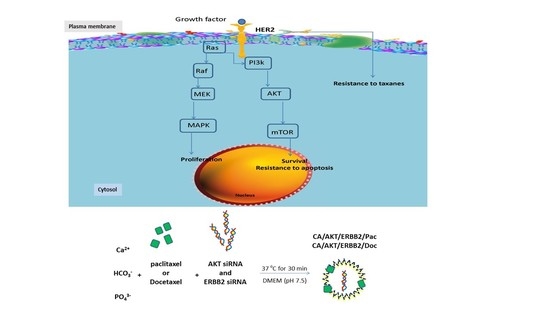
Intracellular Delivery of siRNAs Targeting AKT and ERBB2 Genes Enhances Chemosensitization of Breast Cancer Cells in a Culture and Animal Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Generation and Characterization of Carbonate Apatite
2.4. Complexation of Drugs and siRNAs with Carbonate Apatite
2.5. In Vitro Viability Assay
2.6. Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (Sds-Page) and Western Blot
2.7. Formulation of Particles for In Vivo Study
2.8. 4T1-Induced Breast Cancer Murine Model
2.9. Statistics
3. Results and Discussion
3.1. Cytotoxicity of siRNA-Loaded NPs on MCF-7 and 4T1 Cells
3.2. Effect of CA/siRNA/Drug at the Protein Level
3.3. In Vivo Efficacy of CA/Drug/siRNA
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Holohan, C.; Van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Cancer drug resistance: An evolving paradigm. Nat. Rev. Cancer 2013, 13, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lu, Z.; Zhao, Q.; Huang, J.; Shen, H.; Zhang, Z. Enhanced Chemotherapy Efficacy by Sequential Delivery of siRNA and Anticancer Drugs Using PEI-Grafted Graphene Oxide. Small 2011, 7, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Jung, S.; Luo, S.; Meng, F.; Zhu, X.; Park, T.G.; Zhong, Z. Co-delivery of siRNA and paclitaxel into cancer cells by biodegradable cationic micelles based on PDMAEMA−PCL−PDMAEMA triblock copolymers. Biomaterials 2010, 31, 2408–2416. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhu, W.; Liu, Y.; Yuan, Z.; Yang, S.; Chen, W.; Li, J.; Zhou, X.; Liu, C.; Zhang, X. Novel polymer micelle mediated co-delivery of doxorubicin and P-glycoprotein siRNA for reversal of multidrug resistance and synergistic tumor therapy. Sci. Rep. 2016, 6, 23859. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Liong, M.; Xia, T.; Li, Z.; Ji, Z.; Zink, J.I.; Nel, A.E. Engineered Design of Mesoporous Silica Nanoparticles to Deliver Doxorubicin and P-Glycoprotein siRNA to Overcome Drug Resistance in a Cancer Cell Line. Am. Chem. Soc. Nano 2010, 4, 4539–4550. [Google Scholar] [CrossRef] [PubMed]
- Taratula, O.; Kuzmov, A.; Shah, M.A.; Garbuzenko, O.B.; Minko, T. Nanostructured lipid carriers as multifunctional nanomedicine platform for pulmonary co-delivery of anticancer drugs and siRNA. J. Control. Release 2013, 171, 349–357. [Google Scholar] [CrossRef] [Green Version]
- Meng, H.; Mai, W.X.; Zhang, H.; Xue, M.; Xia, T.; Lin, S.; Wang, X.; Zhao, Y.; Ji, Z.; Zink, J.I.; et al. Codelivery of an Optimal Drug/siRNA Combination Using Mesoporous Silica Nanoparticles to Overcome Drug Resistance in Breast Cancer in Vitro and in Vivo. Am. Chem. Soc. Nano 2013, 7, 994–1005. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; Samy, W.M.; Elgindy, N.A. Protein-based nanocarriers as promising drug and gene delivery systems. J. Control. Release 2012, 161, 38–49. [Google Scholar] [CrossRef]
- Liu, C.F.; Chen, R.; Frezzo, J.A.; Katyal, P.; Hill, L.K.; Yin, L.; Srivastava, N.; More, H.T.; Renfrew, P.D.; Bonneau, R.; et al. Efficient Dual siRNA and Drug Delivery Using Engineered Lipoproteoplexes. Biomacromolecules 2017, 18, 2688–2698. [Google Scholar] [CrossRef]
- Fatemian, T.; Chowdhury, E.H. Cytotoxicity Enhancement in Breast Cancer Cells with Carbonate Apatite-Facilitated Intracellular Delivery of Anti-Cancer Drugs. Toxics 2018, 6, E12. [Google Scholar] [CrossRef]
- Tiash, S.; Chua, M.J.; Chowdhury, E.H. Knockdown of ROS1 gene sensitizes breast tumor growth to doxorubicin in a syngeneic mouse model. Int. J. Oncol. 2016, 48, 2359–2366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiash, S.; Chowdhury, E.H. siRNAs targeting multidrug transporter genes sensitize breast tumor to doxorubicin in a syngeneic mouse model. J. Drug Target. 2018, 27, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Knuefermann, C.; Lu, Y.; Liu, B.; Jin, W.; Liang, K.; Wu, L.; Schmidt, M.; Mills, G.B.; Mendelsohn, J.; Fan, Z. HER2/PI-3K/Akt activation leads to a multidrug resistance in human breast adenocarcinoma cells. Oncogene 2003, 22, 3205–3212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiash, S.; Kamaruzman, N.I.B.; Chowdhury, E.H. Carbonate apatite nanoparticles carry siRNA(s) targeting growth factor receptor genes, EGFR1 and ERBB2 to regress mouse breast tumor. Drug Deliv. 2017, 24, 1721–1730. [Google Scholar] [CrossRef] [PubMed]
- Knowlden, J.M.; Hutcheson, I.R.; Jones, H.E.; Madden, T.; Gee, J.M.; Harper, M.E.; Barrow, D.; Wakeling, A.E.; Nicholson, R.I. Elevated levels of epidermal growth factor receptor/c-erbB2 heterodimers mediate an autocrine growth regulatory pathway in tamoxifen-resistant MCF-7 cells. Endocrinology 2003, 144, 1032–1044. [Google Scholar] [CrossRef]
- Kumar, R.; Mandal, M.; Lipton, A.; Harvey, H.; Thompson, C.B. Overexpression of HER2 modulates bcl-2, bcl-XL, and tamoxifen-induced apoptosis in human MCF-7 breast cancer cells. Clin. Cancer Res. 1996, 2, 1215–1219. [Google Scholar]
- De Iuliis, F.; Salerno, G.; Giuffrida, A.; Milana, B.; Taglieri, L.; Rubinacci, G.; Giantulli, S.; Terella, F.; Silvestri, I.; Scarpa, S. Breast cancer cells respond differently to docetaxel depending on their phenotype and on survivin upregulation. Tumor Biol. 2016, 37, 2603–2611. [Google Scholar] [CrossRef]
- Manandhar, S.; Choi, B.H.; Jung, K.A.; Ryoo, I.G.; Song, M.; Kang, S.J.; Choi, H.G.; Kim, J.A.; Park, P.H.; Kwak, M.K. NRF2 inhibition represses ErbB2 signaling in ovarian carcinoma cells: Implications for tumor growth retardation and docetaxel sensitivity. Free Radic. Biol. Med. 2012, 52, 1773–1785. [Google Scholar] [CrossRef]
- Kim, S.H.; Juhnn, Y.S.; Song, Y.S. Akt Involvement in Paclitaxel Chemoresistance of Human Ovarian Cancer Cells. Ann. N. Y. Acad. Sci. 2007, 1095, 82–89. [Google Scholar] [CrossRef]
- Guo, D.D.; Hong, S.H.; Jiang, H.L.; Kim, J.H.; Minai-Tehrani, A.; Kim, J.E.; Shin, J.Y.; Jiang, T.; Kim, Y.K.; Choi, Y.J.; et al. Synergistic effects of Akt1 shRNA and paclitaxel-incorporated conjugated linoleic acid-coupled poloxamer thermosensitive hydrogel on breast cancer. Biomaterials 2012, 33, 2272–2281. [Google Scholar] [CrossRef]
- Hung, D.Y.; Chie, M. Overexpression of ErbB2 in cancer and ErbB2-targeting strategies. Oncogene 2000, 19, 6115–6121. [Google Scholar] [Green Version]
- Echeverria, C.G.; Sellers, W.R. Drug discovery approaches targeting the PI3K/Akt pathway in cancer. Oncogene 2008, 27, 5511–5526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunz, C.; Borghouts, C.; Buerger, C.; Groner, B. Peptide Aptamers with Binding Specificity for the Intracellular Domain of the ErbB2 Receptor Interfere with AKT Signaling and Sensitize Breast Cancer Cells to Taxol. Mol. Cancer Res. 2006, 4, 983–998. [Google Scholar] [CrossRef] [PubMed]
- Mori, N.; Kyo, S.; Nakamura, M.; Hashimoto, M.; Maida, Y.; Mizumoto, Y.; Takakura, M.; Ohno, S.; Kiyono, T.; Inoue, M. Expression of HER-2 affects patient survival and paclitaxel sensitivity in endometrial cancer. Br. J. Cancer 2010, 103, 889–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orr, G.A.; Verdier-Pinard, P.; McDaid, H.; Horwitz, S.B. Mechanisms of Taxol resistance related to microtubules. Oncogene 2003, 22, 7280–7295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.H.; Liu, H.; Liu, Z.; Ding, Y.; Ledoux, S.P.; Wilson, G.L.; Voellmy, R.; Lin, Y.; Lin, W.; Nahta, R.; et al. Overcoming trastuzumab resistance in breast cancer by targeting dysregulated glucose metabolism. Cancer Res. 2011, 71, 4585–4597. [Google Scholar] [CrossRef]
- Shattuck, D.L.; Miller, J.K.; Carraway, K.L.; Sweeney, C. Met receptor contributes to trastuzumab resistance of Her2-overexpressing breast cancer cells. Cancer Res. 2008, 68, 1471–1477. [Google Scholar] [CrossRef]








| siRNA | Target Sequence | Targeted Gene | Validation Cell Line | % knockdown |
|---|---|---|---|---|
| Hs-AKT1-5 | AATCACACCACCTGACCAAGA | AKT (protein kinase B) | HeLa S3 | 90 |
| Hs_MAPK1_l0 | AAGTTCGAGTAGCTATCAAGA | Mitogen-activated protein kinase | HeLa S3 | 90 |
| Hs-ROS1-5 | AAGGTAATTGCTCTAACTTTA | ROS Proto-Oncogene 1, Receptor tyrosine kinase | HeLa | 87 |
| Hs-ERBB2-14 | AACAAAGAAATCTTAGACGAA | Receptor tyrosine-protein kinase ERBB-2 or human epidermal growth factor receptor 2 (HER2) | MCF-7 | 92 |
| Name | Manufacturer | Molecular Weight | Clonality | Used Dilution |
|---|---|---|---|---|
| AKT (pan) (C67E7) Rabbit mAb | Cell signaling (Danvers, MA, USA) | 60 KDa | Monoclonal | 1:1000 |
| p 44/42 MAPK (Erk 1/2) (137F5) Rabbit mAb | Cell signaling (Danvers, MA, USA) | 42/44 KDa | Monoclonal | 1:1000 |
| ERBB2 (HER2) | Thermo Fisher scientific (Waltham, MA, USA) | 200 KDa | Polyclonal | 1:1000 |
| GAPDH (glyceraldehyde-3-phosphate dehydrogenase) | Cell signaling (Danvers, MA, USA) | 37 KDa | Monoclonal | 1:3000 |
| Group | Regimen |
|---|---|
| Untreated | - |
| CA | 7 µL of 1 M CaCl2 in DMEM |
| CA | 4 µL of 1 M CaCl2 in DMEM |
| Pac | 1.25 mg/kg paclitaxel in DMEM |
| Doc | 1 mg/kg docetaxel in DMEM |
| CA/Pac | 1.25 mg/kg paclitaxel and 7 µL of 1 M CaCl2 in DMEM |
| CA/Pac | 1.25 mg/kg paclitaxel and 4 µL of 1 M CaCl2 in DMEM |
| CA/Doc | 1 mg/kg docetaxel and 4 µL of 1 M CaCl2 in DMEM |
| CA/AKT | 50 nM of AKT siRNA and 4 µL of 1 M CaCl2 in DMEM |
| CA/ERBB2 | 50 nM of ERBB2 siRNA and 4 µL of 1 M CaCl2 in DMEM |
| CA/AKT/ERBB2/Pac | 1.25 mg/kg paclitaxel and 50 nM of ERBB2 and AKT siRNA and 4 µL of 1 M CaCl2 in DMEM |
| CA/AKT/ERBB2/Doc | 1 mg/kg docetaxel and 50 nM of ERBB2 and AKT siRNA and 4 µL of 1 M CaCl2 in DMEM |
| Cell line | Treatment | siRNA Concentration | ||||
|---|---|---|---|---|---|---|
| 1 pM | 10 pM | 100 pM | 1 nM | 10 nM | ||
| 4T1 | CA/ERBB2 | 16.44 ± 5.77 | 33.24 ± 9.14 | 26.70 ± 3.93 | 28.09 ± 6.28 | 19.80 ± 1.13 |
| CA/AKT | 14.11 ± 5.41 | −4.58 ± 5.01 | 3.42 ± 1.23 | −3.27 ± 4.48 | −3.96 ± 2.98 | |
| CA/MAPK | 10.68 ± 3.69 | −0.72 ± 0.87 | 17.59 ± 1.04 | 5.36 ± 1.15 | 25.45 ± 4.64 | |
| CA/ROS1 | 13.85 ± 6.99 | 14.68 ± 3.87 | 27.46 ± 1.17 | 16.65 ± 1.44 | 35.56 ± 2.81 | |
| MCF-7 | CA/ERBB2 | 07.49 ± 8.01 | 24.56 ± 3.19 | 13.82 ± 3.34 | 19.29 ± 3.91 | 8.63 ± 1.67 |
| CA/ROS1 | 12.52 ± 2.79 | 13.69 ± 3.07 | 23.97 ± 2.13 | 28.88 ± 2.29 | 29.70 ± 3.96 | |
| Treatment on MCF-7 Cells | Drug Concentration | ||
|---|---|---|---|
| 10 pM | 100 pM | 1 nM | |
| CA/Pac/AKT | 10.93 ± 1.05 | 8.33 ± 0.84 | 3.31 ± 0.45 |
| CA/Pac/ERBB2 | 7.25 ± 1.91 | 4.11 ± 0.35 | 3.40 ± 1.81 |
| CA/Pac/MAPK | −10.79 ± 2.12 | 1.53 ± 0.55 | 2.06 ± 0.35 |
| CA/Pac/ROS1 | 4.19 ± 1.41 | 3.14 ± 0.74 | 3.64 ± 0.19 |
| CA/Doc/AKT | 5.14 ± 1.22 | 10.45 ± 1.33 | 11.69 ± 2.18 |
| CA/Doc/ERBB2 | 8.95 ± 2.28 | 10.25 ± 0.99 | 14.28 ± 1.04 |
| CA/Doc/MAPK | 3.22 ± 0.91 | 4.27 ± 0.08 | 3.67 ± 1.72 |
| CA/Doc/ROS1 | 1.06 ± 0.59 | 2.83 ± 0.71 | 1.25 ± 0.53 |
| Treatment on 4T1 Cells | Drug Concentration | ||
|---|---|---|---|
| 10 pM | 100 pM | 1 nM | |
| CA/Pac/AKT | −15.93 ± 0.95 | 8.70 ± 2.14 | −2.68 ± 0.54 |
| CA/Pac/ERBB2 | 12.93 ± 0.36 | 9.15 ± 0.14 | 2.42 ± 2.84 |
| CA/Pac/MAPK | −13.79 ± 1.18 | 2.53 ± 0.20 | 2.16 ± 0.11 |
| CA/Pac/ROS1 | 10.09 ± 1.03 | 2.04 ± 0.85 | 7.24 ± 0.07 |
| CA/Doc/AKT | −5.04 ± 0.77 | −9.43 ± 0.51 | 1.30 ± 0.62 |
| CA/Doc/ERBB2 | 5.85 ± 1.08 | 7.21 ± 1.17 | 2.20 ± 1.95 |
| CA/Doc/MAPK | 3.16 ± 0.21 | 5.07 ± 0.98 | 2.67 ± 0.76 |
| CA/Doc/ROS1 | 2.76 ± 0.58 | 1.81 ± 0.97 | 0.05 ± 0.42 |
| CA/Mito/AKT | 0.20 ± 0.71 | 4.97 ± 1.15 | 1.92 ± 0.28 |
| CA/Mito/ERBB2 | 2.13 ± 3.64 | 5.11 ± 0.85 | 1.71 ± 0.38 |
| CA/Mito/MAPK | 1.26 ± 1.23 | 0.23 ± 1.45 | 7.77 ± 0.95 |
| CA/Mito/ROS1 | 0.72 ± 0.92 | 0.52 ± 0.16 | 4.20 ± 1.12 |
| CA/Topo/AKT | 0.02 ± 1.64 | 3.09 ± 0.42 | 5.28 ± 3.07 |
| CA/Topo/ERBB2 | 4.54 ± 2.27 | 5.85 ± 0.47 | 0.86 ± 2.51 |
| CA/Topo/MAPK | 6.54 ± 0.55 | 0.55 ± 0.51 | 4.74 ± 0.88 |
| CA/Topo/ROS1 | 0.58 ± 0.25 | 0.03 ± 0.36 | 0.52 ± 0.81 |
| CA/Pac/AKT/ERBB2 | 19.97 ± 1.73 | 5.48 ± 2.09 | 11.63 ± 2.23 |
| CA/Doc/AKT/ERBB2 | 7.87 ± 1.82 | 1.45 ± 0.37 | 15.16 ± 3.55 |
| CA/Mito/AKT/ERBB2 | 6.04 ± 0.28 | 0.59 ± 0.47 | 4.60 ± 0.82 |
| CA/Topo/AKT/ERBB2 | −10.41 ± 0.66 | −4.73 ± 1.52 | −10.77 ± 0.71 |
| Treatment on MDA-MB-231 Cells | Drug Concentration | ||
|---|---|---|---|
| 10 pM | 100 pM | 1 nM | |
| CA/Pac/AKT | 4.60 ± 1.53 | −8.84 ± 0.92 | 7.55 ± 1.43 |
| CA/Pac/ERBB2 | 12.03 ± 2.44 | 0.59 ± 1.32 | 12.03 ± 3.57 |
| CA/Pac/MAPK | 8.96 ± 2.16 | 0.35 ± 0.36 | 8.25 ± 1.28 |
| CA/Pac/ROS1 | 11.08 ± 2.09 | 0.35 ± 0.09 | 7.90 ± 1.83 |
| CA/Doc/AKT | 3.66 ± 0.24 | 8.97 ± 2.94 | 16.16 ± 4.52 |
| CA/Doc/ERBB2 | 20.05 ± 4.61 | 7.97 ± 3.06 | 8.02 ± 1.49 |
| CA/Doc/MAPK | −1.06 ± 0.58 | 6.14 ± 2.73 | 6.49 ± 1.91 |
| CA/Doc/ROS1 | 0.59 ± 0.34 | 10.74 ± 3.16 | 7.90 ± 1.52 |
| CA/Pac/AKT/ERBB2 | 7.07 ± 2.22 | 3.71 ± 0.85 | 13.29 ± 1.12 |
| CA/Pac/AKT/MAPK | 18.62 ± 3.64 | 14.42 ± 2.14 | 3.37 ± 3.47 |
| CA/Pac/AKT/ROS1 | 11.26 ± 1.05 | −0.12 ± 0.93 | 22.40 ± 3.44 |
| CA/Pac/ERBB2/MAPK | 1.32 ± 0.07 | −1.44 ± 0.12 | 12.22 ± 2.33 |
| CA/Pac/ERBB2/ROS1 | −0.48 ± 0.52 | −9.70 ± 0.49 | 5.51 ± 1.86 |
| CA/Pac/MAPK/ROS1 | 9.58 ± 1.24 | −4.79 ± 0.93 | 6.23 ± 2.22 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fatemian, T.; Moghimi, H.R.; Chowdhury, E.H. Intracellular Delivery of siRNAs Targeting AKT and ERBB2 Genes Enhances Chemosensitization of Breast Cancer Cells in a Culture and Animal Model. Pharmaceutics 2019, 11, 458. https://doi.org/10.3390/pharmaceutics11090458
Fatemian T, Moghimi HR, Chowdhury EH. Intracellular Delivery of siRNAs Targeting AKT and ERBB2 Genes Enhances Chemosensitization of Breast Cancer Cells in a Culture and Animal Model. Pharmaceutics. 2019; 11(9):458. https://doi.org/10.3390/pharmaceutics11090458
Chicago/Turabian StyleFatemian, Tahereh, Hamid Reza Moghimi, and Ezharul Hoque Chowdhury. 2019. "Intracellular Delivery of siRNAs Targeting AKT and ERBB2 Genes Enhances Chemosensitization of Breast Cancer Cells in a Culture and Animal Model" Pharmaceutics 11, no. 9: 458. https://doi.org/10.3390/pharmaceutics11090458