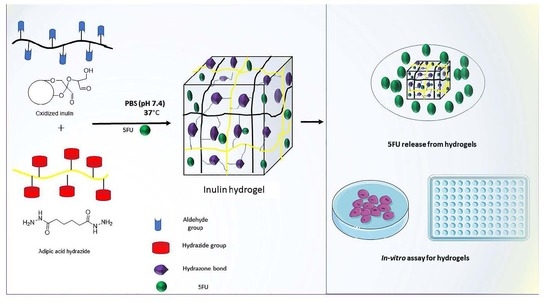
Preparation and Characterization of Oxidized Inulin Hydrogel for Controlled Drug Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Oxidation of Inulin to Inulin Aldehyde Derivative
Preparation and Characterization of Oxidized Inulin
2.3. Determination of the Degree of Oxidation (Aldehyde Content Analysis)
2.3.1. Determination of Degree of Oxidation
Hydroxylamine Titration
1H NMR Spectroscopy after Titration with tBC
2.4. Preparation and Characterization of Hydrogels (Wissembourg, France)
2.5. Characterization of Oxidized Polymer and Hydrogels: FTIR and 1H NMR
2.6. Characterization of Hydrogels: TGA
2.7. Characterization of Hydrogels: DSC
2.8. Rheological Evaluation
2.9. Scanning Electron Microscope (SEM) Analysis
2.10. Dynamic Swelling Experiments: Swelling Analysis
2.11. Release Kinetics of 5FU from Crosslinked Hydrogels
2.12. Degradation Studies
2.13. MTT Assay
3. Results and Discussion
3.1. Oxidized Inulin Characterization
3.2. FTIR
3.3. SEM
3.4. TGA/First derivative of the TGA Curve (DTG)
3.5. DSC
3.6. Rheological Properties
3.7. Gelation and Swelling Studies
3.8. Release Kinetics of 5FU from Crosslinked Hydrogels
3.9. Degradation
3.10. Cytotoxicity
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Mensink, M.A.; Frijlink, H.W.; Maarschalk, K.V.; Hinrichs, W.L. Inulin, a flexible oligosaccharide I: Review of its physicochemical characteristics. Carbohydr. Polym. 2015, 130, 405–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barclay, T.; Ginic-Markovic, M.; Cooper, P.; Petrovsky, N. Inulin—A versatile polysaccharide with multiple pharmaceutical and food chemical uses. J. Excip. Food Chem. 2010, 1, 27–50. [Google Scholar]
- Flamm, G.; Glinsmann, W.; Kritchevsky, D.; Prosky, L.; Roberfroid, M. Inulin and oligofructose as dietary fiber: A review of the evidence. Crit. Rev. Food Sci. Nutr. 2001, 41, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Roberfroid, M.B.; Delzenne, N.M. Dietary fructans. Annu. Rev. Nutr. 1998, 18, 117–143. [Google Scholar] [CrossRef] [PubMed]
- Maris, B.; Verheyden, L.; van Reeth, K.; Samyn, C.; Augustijns, P.; Kinget, R.; van den Mooter, G. Synthesis and characterisation of inulin-azo hydrogels designed for colon targeting. Int. J. Pharm. 2001, 213, 143–152. [Google Scholar] [CrossRef]
- Keenan, D.F.; Resconi, V.C.; Kerry, J.P.; Hamill, R.M. Modelling the influence of inulin as a fat substitute in comminuted meat products on their physico-chemical characteristics and eating quality using a mixture design approach. Meat Sci. 2014, 96, 1384–1394. [Google Scholar] [CrossRef] [PubMed]
- Furlan, L.T.R.; Padilla, A.P.; Campderros, M.E. Development of reduced fat minced meats using inulin and bovine plasma proteins as fat replacers. Meat Sci. 2014, 96, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Poulain, N.; Dez, I.; Perrio, C.; Lasne, M.C.; Prud’homme, M.P.; Nakache, E. Microspheres based on inulin for the controlled release of serine protease inhibitors: Preparation, characterization and in vitro release. J. Control Release 2003, 92, 27–38. [Google Scholar] [CrossRef]
- Lopez-Molina, D.; Chazarra, S.; How, C.W.; Pruidze, N.; Navarro-Peran, E.; Garcia-Canovas, F.; Garcia-Ruiz, P.A.; Rojas-Melgarejo, F.; Rodriguez-Lopez, J.N. Cinnamate of inulin as a vehicle for delivery of colonic drugs. Int. J. Pharm. 2015, 479, 96–102. [Google Scholar] [CrossRef]
- Vervoort, L.; van den Mooter, G.; Augustijns, P.; Busson, R.; Toppet, S.; Kinget, R. Inulin hydrogels as carriers for colonic drug targeting: I. Synthesis and characterization of methacrylated inulin and hydrogen formation. Pharm. Res. 1997, 14, 1730–1737. [Google Scholar] [CrossRef]
- Vervoort, L.; Vinckier, I.; Moldenaers, P.; van den Mooter, G.; Augustijns, P.; Kinget, R. Inulin hydrogels as carriers for colonic drug targeting. Rheological characterization of the hydrogel formation and the hydrogel network. J. Pharm. Sci. 1999, 8, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Sood, V.; Bora, M.; Vasita, R.; Katti, D.S. Electrosprayed inulin microparticles for microbiota triggered targeting of colon. Carbohydr. Polym. 2014, 112, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Zijlstra, G.S.; Ponsioen, B.J.; Hummel, S.A.; Sanders, N.; Hinrichs, W.L.; De Boer, A.H.; Frijlink, H.W. Formulation and process development of (recombinant human) deoxyribonuclease I as a powder for inhalation. Pharm. Dev. Technol. 2009, 14, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Rahul, R.; Jha, U.; Sen, G.; Mishra, S. Carboxymethyl inulin: A novel flocculant for wastewater treatment. Int. J. Biol. Macromol. 2014, 63, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, F.S.; Fiorica, C.; Di Stefano, M.; Pitarresi, G.; Gulino, A.; Agnello, S.; Giammona, G. In situ forming hydrogels of hyaluronic acid and inulin derivatives for cartilage regeneration. Carbohydr. Polym. 2015, 122, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Afinjuomo, F.; Barclay, T.G.; Song, Y.; Parikh, A.; Petrovsky, N.; Garg, S.; Parikh, A. Synthesis and characterization of a novel inulin hydrogel crosslinked with pyromellitic dianhydride. React. Funct. Polym. 2019, 134, 104–111. [Google Scholar] [CrossRef]
- Mooter, G.V.D.; Vervoort, L.; Kinget, R. Characterization of methacrylated inulin hydrogels designed for colon targeting: In vitro release of BSA. Pharm. Res. 2003, 20, 303–307. [Google Scholar] [CrossRef]
- Tripodo, G.; Pitarresi, G.; Cavallaro, G.; Palumbo, F.S.; Giammona, G. Controlled release of IgG by novel UV induced polysaccharide/poly(amino acid) hydrogels. Macromol. Biosci. 2009, 9, 393–401. [Google Scholar] [CrossRef]
- Castelli, F.; Sarpietro, M.G.; Micieli, D.; Ottimo, S.; Pitarresi, G.; Tripodo, G.; Carlisi, B.; Giammona, G. Differential scanning calorimetry study on drug release from an inulin-based hydrogel and its interaction with a biomembrane model: pH and loading effect. Eur. J. Pharm. Sci. 2008, 35, 76–85. [Google Scholar] [CrossRef]
- Licciardi, M.; Scialabba, C.; Sardo, C.; Cavallaro, G.; Giammona, G. Amphiphilic inulin graft co-polymers as self-assembling micelles for doxorubicin delivery. J. Mater. Chem. B 2014, 2, 4262–4271. [Google Scholar] [CrossRef]
- Muley, P.; Kumar, S.; El Kourati, F.; Kesharwani, S.S.; Tummala, H. Hydrophobically modified inulin as an amphiphilic carbohydrate polymer for micellar delivery of paclitaxel for intravenous route. Int. J. Pharm. 2016, 500, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Mandracchia, D.; Rosato, A.; Trapani, A.; Chlapanidas, T.; Montagner, I.M.; Perteghella, S.; Di Franco, C.; Torre, M.L.; Trapani, G.; Tripodo, G. Design, synthesis and evaluation of biotin decorated inulin-based polymeric micelles as long-circulating nanocarriers for targeted drug delivery. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1245–1254. [Google Scholar] [CrossRef] [PubMed]
- Essien, H.; Lai, J.Y.; Hwang, K.J. Synthesis of diethylenetriaminepentaacetic acid conjugated inulin and utility for cellular uptake of liposomes. J. Med. Chem. 1988, 31, 898–901. [Google Scholar] [CrossRef] [PubMed]
- Volsi, A.L.; de Aberasturi, D.J.; Henriksen-Lacey, M.; Giammona, G.; Licciardi, M.; Liz-Marzan, L.M.Y. Inulin coated plasmonic gold nanoparticles as a tumor-selective tool for cancer therapy. J. Mater. Chem. B 2016, 4, 1150–1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Li, Y.; Wang, C.; Li, G.; Zhao, Y.; Yang, Y. Synthesis of methylprednisolone loaded ibuprofen modified inulin based nanoparticles and their application for drug delivery. Mater. Sci. Eng. C 2014, 42, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Licciardi, M.; Volsi, A.L.; Mauro, N.; Scialabba, C.; Cavallaro, G.; Giammona, G. Preparation and characterization of inulin coated gold nanoparticles for selective delivery of doxorubicin to breast cancer cells. J. Nanomater. 2016, 2016, 1–12. [Google Scholar] [CrossRef]
- Silva, D.G.; Cooper, P.D.; Petrovsky, N. Inulin-derived adjuvants efficiently promote both Th1 and Th2 immune responses. Immunol. Cell Biol. 2004, 82, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Cooper, P.D.; Petrovsky, N. Delta inulin: A novel, immunologically active, stable packing structure comprising beta-D-[2 -> 1] poly(fructo-furanosyl) alpha-D-glucose polymers. Glycobiology 2011, 21, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Tummala, H. Development of soluble inulin microparticles as a potent and safe vaccine adjuvant and delivery system. Mol. Pharm. 2013, 10, 1845–1853. [Google Scholar] [CrossRef] [PubMed]
- Srinarong, P.; Hamalainen, S.; Visser, M.R.; Hinrichs, W.L.; Ketolainen, J.; Frijlink, H.W. Surface-active derivative of inulin (Inutec(R) SP1) is a superior carrier for solid dispersions with a high drug load. J. Pharm. Sci. 2011, 100, 2333–2342. [Google Scholar] [CrossRef] [PubMed]
- Fares, M.M.; Salem, M.S.; Khanfar, M. Inulin and poly(acrylic acid) grafted inulin for dissolution enhancement and preliminary controlled release of poorly water-soluble Irbesartan drug. Int. J. Pharm. 2011, 410, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Robert, P.; Garcia, P.; Reyes, N.; Chávez, J.; Santos, J. Acetylated starch and inulin as encapsulating agents of gallic acid and their release behaviour in a hydrophilic system. Food Chem. 2012, 134, 1–8. [Google Scholar] [CrossRef]
- Schacht, E.; Ruys, L.; Vermeersch, J.; Remon, J.P.; Duncan, R. Use of polysaccharides as drug carriers. Dextran and inulin derivatives of procainamide. Ann. N. Y. Acad. Sci. 1985, 446, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Hartzell, A.L.; Maldonado-Gómez, M.X.; Yang, J.; Hutkins, R.W.; Rose, D.J. In vitro digestion and fermentation of 5-formyl-aminosailcylate-inulin: A potential prodrug of 5-aminosalicylic acid. Bioact. Carbohydr. Diet. Fibre 2013, 2, 8–14. [Google Scholar] [CrossRef]
- Sahiner, N.; Sagbas, S.; Yoshida, H.; Lyon, L.A. Synthesis and properties of inulin based microgels. Coll. Interface Sci. Commun. 2014, 2, 15–18. [Google Scholar] [CrossRef]
- Mandracchia, D.; Denora, N.; Franco, M.; Pitarresi, G.; Giammona, G.; Trapani, G. New biodegradable hydrogels based on inulin and alpha,beta-polyaspartylhydrazide designed for colonic drug delivery: In vitro release of glutathione and oxytocin. J. Biomater. Sci. Polym. Ed. 2011, 2, 313–328. [Google Scholar] [CrossRef] [PubMed]
- Pitarresi, G.; Tripodo, G.; Calabrese, R.; Craparo, E.F.; Licciardi, M.; Giammona, G. Hydrogels for potential colon drug release by thiol-ene conjugate addition of a new inulin derivative. Macromol. Biosci. 2008, 8, 891–902. [Google Scholar] [CrossRef]
- Chiu, H.; Hsu, Y.; Lin, P. Synthesis of pH-sensitive inulin hydrogels and characterization of their swelling properties. J. Biomed. Mater. Res. 2002, 61, 146–152. [Google Scholar] [CrossRef]
- Chen, S.; Cui, S.; Zhang, H.; Pei, X.; Hu, J.; Zhou, Y.Z.; Liu, Y. Cross-linked pectin nanofibers with enhanced cell adhesion. Biomacromolecules 2018, 19, 490–498. [Google Scholar] [CrossRef]
- Gupta, B.; Tummalapalli, M.; Deopura, B.; Alam, M.S. Functionalization of pectin by periodate oxidation. Carbohydr. Polym. 2013, 98, 1160–1165. [Google Scholar] [CrossRef]
- Maia, J.; Ferreira, L.; Carvalho, R.; Ramos, M.A.; Gil, M.H. Synthesis and characterization of new injectable and degradable dextran-based hydrogels. Polymer 2005, 46, 9604–9614. [Google Scholar] [CrossRef] [Green Version]
- Cai, M.; Gong, J.; Cao, J.; Chen, Y.; Luo, X. In situ chemically crosslinked chitosan membrane by adipic acid. J. Appl. Polym. Sci. 2013, 128, 3308–3314. [Google Scholar] [CrossRef]
- Maiti, S.; Singha, K.; Ray, S.; Dey, P.; Sa, B. Adipic acid dihydrazide treated partially oxidized alginate beads for sustained oral delivery of flurbiprofen. Pharm. Dev. Technol. 2009, 14, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Paşcalău, V.; Popescu, V.; Popescu, G.L.; Dudescu, M.C.; Borodi, G.; Dinescu, A.M.; Moldovan, M. Obtaining and characterizing alginate/k-carrageenan hydrogel cross-linked with adipic dihydrazide. Adv. Mater. Sci. Eng. 2013, 2013, 1–12. [Google Scholar] [CrossRef]
- Hu, M.H.; Yang, K.C.; Sun, Y.H.; Chen, Y.C.; Yang, S.H.; Lin, F.H. In situ forming oxidised hyaluronic acid/adipic acid dihydrazide hydrogel for prevention of epidural fibrosis after laminectomy. Eur. Cells Mater. 2017, 34, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Shoham, N.; Sasson, A.L.; Lin, F.-H.; Benayahu, D.; Haj-Ali, R.; Gefen, A. The mechanics of hyaluronic acid/adipic acid dihydrazide hydrogel: Towards developing a vessel for delivery of preadipocytes to native tissues. J. Mech. Behav. Biomed. Mater. 2013, 28, 320–331. [Google Scholar] [CrossRef]
- Su, W.-Y.; Chen, K.-H.; Chen, Y.-C.; Lee, Y.-H.; Tseng, C.-L.; Lin, F.-H. An injectable oxidated hyaluronic acid/adipic acid dihydrazide hydrogel as a vitreous substitute. J. Biomater. Sci. Polym. Ed. 2011, 22, 1777–1797. [Google Scholar] [CrossRef]
- Su, W.-Y.; Chen, Y.-C.; Lin, F.-H. Injectable oxidized hyaluronic acid/adipic acid dihydrazide hydrogel for nucleus pulposus regeneration. Acta Biomater. 2010, 6, 3044–3055. [Google Scholar] [CrossRef]
- Tabandeh, M.R.; Aminlari, M. Synthesis, physicochemical and immunological properties of oxidized inulin–l-asparaginase bioconjugate. J. Biotechnol. 2009, 141, 189–195. [Google Scholar] [CrossRef]
- Barclay, T.; Ginic-Markovic, M.; Johnston, M.R.; Cooper, P.D.; Petrovsky, N. Analysis of the hydrolysis of inulin using real time 1H NMR spectroscopy. Carbohydr. Res. 2012, 352, 117–125. [Google Scholar] [CrossRef] [Green Version]
- Schacht, E.; Vermeersch, J.; Vandoorne, F.; Vercauteren, R.; Remon, J. Synthesis and characterization of some modified polysaccharides containing drug moieties. J. Control. Release 1985, 2, 245–256. [Google Scholar] [CrossRef]
- Zhang, K.; Yan, S.; Wang, T.; Feng, L.; Zhu, J.; Chen, X.; Cui, L.; Yin, J. Injectable in situ self-cross-linking hydrogels based on poly( l -glutamic acid) and alginate for cartilage tissue engineering. Biomacromolecules 2014, 15, 4495–4508. [Google Scholar]
- Zhao, H.; Heindel, N.D. Determination of degree of substitution of formyl groups in polyaldehyde dextran by the hydroxylamine hydrochloride method. Pharm. Res. 1991, 8, 400–402. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Burdick, J.A.; Kobler, J.; Clifton, R.J.; Rosowski, J.J.; Zeitels, S.M.; Langer, R. Synthesis and characterization of in situ cross-linkable hyaluronic acid-based hydrogels with potential application for vocal fold regeneration. Macromolecules 2004, 37, 3239–3248. [Google Scholar] [CrossRef]
- De Mattos, A.C.; Khalil, N.M.; Mainardes, R. Development and validation of an HPLC method for the determination of fluorouracil in polymeric nanoparticles. Braz. J. Pharm. Sci. 2013, 49, 117–126. [Google Scholar] [CrossRef] [Green Version]
- Vervoort, L.; Rombaut, P.; van den Mooter, G.; Augustijns, P.; Kinget, R. Inulin hydrogels. II. In vitro degradation study. Int. J. Pharm. 1998, 172, 137–145. [Google Scholar] [CrossRef]
- Damian, F.; Mooter, G.V.D.; Samyn, C.; Kinget, R. In vitro biodegradation study of acetyl and methyl inulins by Bifidobacteria and inulinase. Eur. J. Pharm. Biopharm. 1999, 47, 275–282. [Google Scholar] [CrossRef]
- Pitarresi, G.; Tripodo, G.; Cavallaro, G.; Palumbo, F.S.; Giammona, G. Inulin–iron complexes: A potential treatment of iron deficiency anaemia. Eur. J. Pharm. Biopharm. 2008, 68, 267–276. [Google Scholar] [CrossRef]
- Prabaharan, M.; Grailer, J.J.; Pilla, S.; Steeber, D.A.; Gong, S. Amphiphilic multi-arm block copolymer based on hyperbranched polyester, poly(L-lactide) and poly(ethylene glycol) as a drug delivery carrier. Macromol. Biosci. 2009, 9, 515–524. [Google Scholar] [CrossRef]
- Seeli, D.S.; Prabaharan, M. Guar gum oleate-graft-poly(methacrylic acid) hydrogel as a colon-specific controlled drug delivery carrier. Carbohydr. Polym. 2017, 158, 51–57. [Google Scholar] [CrossRef]
- Parikh, A.; Kathawala, K.; Li, J.; Chen, C.; Shan, Z.; Cao, X.; Zhou, X.-F.; Garg, S. Curcumin-loaded self-nanomicellizing solid dispersion system: Part II: In vivo safety and efficacy assessment against behavior deficit in Alzheimer disease. Drug Deliv. Transl. Res. 2018, 8, 1406–1420. [Google Scholar] [CrossRef] [PubMed]
- Stevens, C.V.; Meriggi, A.; Booten, K. Chemical modification of inulin, a valuable renewable resource, and its industrial applications. Biomacromolecules 2001, 2, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ishak, M.F.; Painter, T. Kinetic evidence for hemiacetal formation during the oxidation of dextran in aqueous periodate. Carbohydr. Res. 1978, 64, 189–197. [Google Scholar]
- Pan, J.-F.; Yuan, L.; Guo, C.-A.; Geng, X.-H.; Fei, T.; Fan, W.-S.; Li, S.; Yuan, H.-F.; Yan, Z.-Q.; Mo, X.-M. Fabrication of modified dextran–gelatin in situ forming hydrogel and application in cartilage tissue engineering. J. Mater. Chem. B 2014, 2, 8346–8360. [Google Scholar] [CrossRef]
- Kim, U.-J.; Kuga, S.; Wada, M.; Okano, T.; Kondo, T. Periodate oxidation of crystalline cellulose. Biomacromolecules 2000, 1, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Gomez, C.; Rinaudo, M.; Villar, M.; Gomez, C. Oxidation of sodium alginate and characterization of the oxidized derivatives. Carbohydr. Polym. 2007, 67, 296–304. [Google Scholar] [CrossRef]
- Kristiansen, K.A.; Potthast, A.; Christensen, B.E. Periodate oxidation of polysaccharides for modification of chemical and physical properties. Carbohydr. Res. 2010, 345, 1264–1271. [Google Scholar] [CrossRef] [PubMed]
- Maia, J.; Ribeiro, M.P.; Ventura, C.; Carvalho, R.A.; Correia, I.J.; Gil, M.H. Ocular injectable formulation assessment for oxidized dextran-based hydrogels. Acta Biomater. 2009, 5, 1948–1955. [Google Scholar] [CrossRef] [Green Version]
- Bouhadir, K.H.; Hausman, D.S.; Mooney, D.J. Synthesis of cross-linked poly(aldehyde guluronate) hydrogels. Polymer 1999, 40, 3575–3584. [Google Scholar] [CrossRef]
- Li, X.; Xu, A.; Xie, H.; Yu, W.; Xie, W.; Ma, X. Preparation of low molecular weight alginate by hydrogen peroxide depolymerization for tissue engineering. Carbohydr. Polym. 2010, 79, 660–664. [Google Scholar] [CrossRef]
- Villanueva-Carcia, D.N.; Rangel-Vazquez, N.A.; Kalla, J. Structural analysis of adsorption processes of 5FU and imiquimod on hydrogels using AMBER/PM3 hybrid model. Rev. Colomb. Quím. 2018, 47, 28–35. [Google Scholar]
- Miralinaghi, P.; Kashani, P.; Moniri, E.; Miralinaghi, M.; Monir, E.; Miralinaghi, M. Non-linear kinetic, equilibrium, and thermodynamic studies of 5-fluorouracil adsorption onto chitosan–functionalized graphene oxide. Mater. Res. Express 2019, 6, 65305. [Google Scholar] [CrossRef]
- Dan, A.; Ghosh, S.; Moulik, S.P. Physicochemical studies on the biopolymer inulin: A critical evaluation of its self-aggregation, aggregate-morphology, interaction with water, and thermal stability. Biopolymers 2009, 91, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Bouhadir, K.; Lee, K.; Alsberg, E.; Damm, K.; Anderson, K.; Mooney, D. Degradation of partially oxidized alginate and its potential application for tissue engineering. Biotechnol. Prog. 2001, 17, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Maia, J.; Carvalho, R.A.; Coelho, J.F.; Simões, P.N.N.; Gil, M.H. Insight on the periodate oxidation of dextran and its structural vicissitudes. Polymer 2011, 52, 258–265. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Jayakrishnan, A. Self-cross-linking biopolymers as injectable in situ forming biodegradable scaffolds. Biomaterials 2005, 26, 3941–3951. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Periodate oxidation of methylcellulose: Characterization and properties of oxidized derivatives. Polymer 2010, 2, 505–521. [Google Scholar] [CrossRef]
- Mitra, T.; Sailakshmi, G.; Gnanamani, A.; Mandal, A.B. Adipic acid interaction enhances the mechanical and thermal stability of natural polymers. J. Appl. Polym. Sci. 2012, 125. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Su, W.-Y.; Yang, S.-H.; Gefen, A.; Lin, F.-H. In situ forming hydrogels composed of oxidized high molecular weight hyaluronic acid and gelatin for nucleus pulposus regeneration. Acta Biomater. 2013, 9, 5181–5193. [Google Scholar] [CrossRef]
- Zhu, R.; Chen, R.; Duo, Y.; Zhang, S.; Xie, D.; Mei, Y. An industrial scale synthesis of adipicdihydrazide (ADH)/polyacrylate hybrid with excellent formaldehyde degradation performance. Polymer 2019, 11, 86. [Google Scholar] [CrossRef]
- Larrañeta, E.; Henry, M.; Irwin, N.J.; Trotter, J.; Perminova, A.A.; Donnelly, R.F. Synthesis and characterization of hyaluronic acid hydrogels crosslinked using a solvent-free process for potential biomedical applications. Carbohydr. Polym. 2018, 181, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Zhang, L.-M.; Li, N.-N.; Shi, J.-F. In situ crosslinkable hydrogel formed from a polysaccharide-based hydrogelator. Biomacromolecules 2009, 10, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Mohana, M.; Muthiah, P.; McMillen, C. Supramolecular hydrogen-bonding patterns in 1:1 cocrystals of 5-fluorouracil with 4-methylbenzoic acid and 3-nitrobenzoic acid. Acta Crystallogr. Sect. C Struct. Chem. 2017, 73, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Anirudhan, T.S.; Nima, J.; Divya, P.L. Synthesis, characterization and in vitro cytotoxicity analysis of a novel cellulose based drug carrier for the controlled delivery of 5-fluorouracil, an anticancer drug. Appl. Surf. Sci. 2015, 355, 64–73. [Google Scholar] [CrossRef]









| Oxidized Inulin | The Degree of Oxidation by Hydroxylamine (%) a | The Degree of Oxidation by NMR (%) b |
|---|---|---|
| Oxi-2h | 25.6 ± 0.6 | 23 ± 0.9 |
| Oxi-3h | 31 ± 0.8 | 26 ± 0.7 |
| Oxi-4h | 34 ± 0.3 | 31 ± 1.2 |
| Oxi-15h | 38 ± 0.4 | n |
| Oxi-20h | 43.6 ± 0.5 | n |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afinjuomo, F.; Fouladian, P.; Parikh, A.; Barclay, T.G.; Song, Y.; Garg, S. Preparation and Characterization of Oxidized Inulin Hydrogel for Controlled Drug Delivery. Pharmaceutics 2019, 11, 356. https://doi.org/10.3390/pharmaceutics11070356
Afinjuomo F, Fouladian P, Parikh A, Barclay TG, Song Y, Garg S. Preparation and Characterization of Oxidized Inulin Hydrogel for Controlled Drug Delivery. Pharmaceutics. 2019; 11(7):356. https://doi.org/10.3390/pharmaceutics11070356
Chicago/Turabian StyleAfinjuomo, Franklin, Paris Fouladian, Ankit Parikh, Thomas G. Barclay, Yunmei Song, and Sanjay Garg. 2019. "Preparation and Characterization of Oxidized Inulin Hydrogel for Controlled Drug Delivery" Pharmaceutics 11, no. 7: 356. https://doi.org/10.3390/pharmaceutics11070356