β-Cyclodextrin as a Functional Excipient Used for Enhancing the Diminazene Aceturate Bioavailability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Solid Inclusion Complex Preparation
2.3. Stoichiometry of the Inclusion Complex and Association Constant Determination
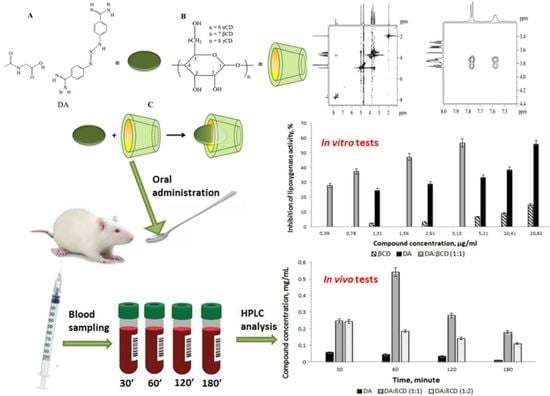
2.4. Physico-Chemical Characterization of DA:βCD Inclusion Complexes
2.5. DA Calibration Curve
2.6. Stability Studies of DA and DA:βCD (1:1) in Acidic and Neutral pH
2.7. Antioxidant Activity of the DA:βCD (1:1) Inclusion Complex
2.8. Evaluation of the Drug Release Profile in Blood Plasma from Treated Rats
2.8.1. Animals
2.8.2. Experimental Design
2.8.3. HPLC Determination of the Diminazene Aceturate (DA) from Rats’ Plasma
2.8.4. Blood Samples Preparation
2.9. Statistical Analysis
3. Results and Discussion
3.1. Physico-Chemical Characterization of the DA:βCD (1:1) Inclusion Complex
3.1.1. Raman Analysis of the DA:βCD (1:1) Inclusion Complex
3.1.2. DSC Analysis of the DA:βCD (1:1) Inclusion Complex
3.1.3. Wide-Angle X ray Diffraction (WAXD) Analysis of the DA:βCD (1:1) Inclusion Complex
3.1.4. NMR Spectroscopy Analysis of DA:βCD (1:1) Inclusion Complex
3.2. Chemical Stability of DA in Acidic and Neutral pH from DA and DA:βCD (1:1) Aqueous Solutions
3.3. Biological Activity Determination
3.3.1. Antioxidant Activity of the DA:βCD (1:1) Inclusion Complex
3.3.2. Evaluation of the Drug Release Profile in Blood Plasma from Treated Rats
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CDs | cyclodextrins |
| βCD | beta-cyclodextrin |
| DA | diminazene aceturate |
| DA:βCD (1:1) | inclusion complex in 1:1 molar ratio of components |
| DA:βCD (1:2) | lyophilized mixture in 1:2 molar ratio of components |
| 1H-NMR | Proton Nuclear Magnetic Resonance |
| H-H ROESY | Rotating Frame Overhause Effect |
| DSC | Differential Scanning Calorimetry |
| HPLC | High Performance Liquid Chromatography |
| WAXD | Wide angle X-ray Diffraction |
References
- Garg, R. Supramolecular Chemistry of Host-Guest Inclusion Complexes; GRIN Verlag: Munich, Germany, 2012; Available online: https://www.grin.com/document/187894 (accessed on 16 May 2019).
- Corciova, A.; Ciobanu, C.; Poiata, A.; Nicolescu, A.; Drobota, M.; Varganici, C.D.; Pinteala, T.; Fifere, A.; Marangoci, N.; Mircea, C. Inclusion complexes of hesperidin with hydroxypropyl-β-cyclodextrin. Physico-chemical characterization and biological assessment. Dig. J. Nanomater. Bios. 2014, 9, 1623–1637. [Google Scholar]
- Corciova, A.; Ciobanu, C.; Poiata, A.; Mircea, C.; Nicolescu, A.; Drobota, M.; Varganici, C.D.; Pinteala, T.; Marangoci, N. Antibacterial and antioxidant properties of hesperidin: Beta-cyclodextrin complexes obtained by different techniques. J. Incl. Phenom. Macrocycl. Chem. 2015, 81, 71–84. [Google Scholar] [CrossRef]
- Han, Y.; Liu, W.; Huang, J.; Qiu, S.; Zhong, H.; Liu, D.; Liu, J. Cyclodextrin-based metal-organic frameworks (CD-MOFs) in pharmaceutics and biomedicine. Pharmaceutics 2018, 10, 271. [Google Scholar] [CrossRef] [PubMed]
- Marangoci, N.; Mares, M.; Silion, M.; Fifere, A.; Varganici, C.; Nicolescu, A.; Deleanu, C.; Coroaba, A.; Pinteala, M.; Simionescu, B.C. Inclusion complex of a new propiconazole derivative with β-cyclodextrin: NMR, ESI–MS and preliminary pharmacological studies. Res. Pharm. Sci. 2011, 1, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Jiang, M. Cyclodextrin-based inclusion complexation bridging supramolecular chemistry and macromolecular self-assembly. Chem. Soc. Rev. 2011, 40, 2254–2266. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, A.J.; Singh, S.; Chhipa, R.R.; Vijayakumar, M.V.; Ajay, A.K.; Bhat, M.K. Methyl-β-cyclodextrin enhances the susceptibility of human breast cancer cells to carboplatin and 5-fluorouracil: Involvement of Akt, NF-κB and Bcl-2. Toxicol. Appl. Pharmacol. 2006, 216, 177–185. [Google Scholar] [CrossRef]
- Zarzycki, P.K.; Fenert, B.; Głód, B.K. Cyclodextrins-based nanocomplexes for encapsulation of bioactive compounds in food, cosmetics, and pharmaceutical products: Principles of supramolecular complexes formation, their influence on the antioxidative properties of target chemicals, and recent advances in selected industrial applications. In Encapsulations, Nanotechnology in the Agri-Food Industry; Grumezescu, A.M., Ed.; Academic Press, Elsevier Science Publishing Co Inc.: Cambridge, MA, USA, 2016; Volume 2, pp. 717–767. [Google Scholar]
- Kuriakose, S.; Uzonna, J.E. Diminazene aceturate (Berenil), a new use for an old compound? Int. Immunopharmacol. 2014, 21, 342–345. [Google Scholar] [CrossRef]
- da Silva Oliveira, G.L.; de Freitas, R.M. Diminazene aceturate—An antiparasitic drug of antiquity: Advances in pharmacology & therapeutics. Pharmacol. Res. 2015, 102, 138–157. [Google Scholar] [CrossRef]
- Dhawale, V.S.; Amara, V.R.; Karpe, P.A.; Malek, V.; Patel, D.; Tikoo, K. Activation of angiotensin-converting enzyme 2 (ACE2) attenuates allergic airway inflammation in rat asthma model. Toxicol. Appl. Pharmacol. 2016, 306, 17–26. [Google Scholar] [CrossRef]
- Souza, L.K.; Nicolau, L.A.; Sousa, N.A.; Araujo, T.S.; Sousa, F.B.; Costa, D.S.; Souza, F.M.; Pacifico, D.M.; Martins, C.S.; Silva, R.O.; et al. Diminazene aceturate, an angiotensin-converting enzyme II activator, prevents gastric mucosal damage in mice: Role of the angiotensin-(1–7)/mas receptor axis. Biochem. Pharmacol. 2016, 112, 50–59. [Google Scholar] [CrossRef]
- Bennion, D.M.; Haltigan, E.A.; Irwin, A.J.; Donnangelo, L.L.; Regenhardt, R.W.; Pioquinto, D.J.; Purich, D.L.; Sumners, C. Activation of the neuroprotective angiotensin-converting enzyme 2 in rat ischemic stroke. Hypertension 2015, 66, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Shil, P.K.; Zhu, P.; Yang, H.; Verma, A.; Lei, B.; Li, Q. Angiotensin-converting enzyme 2 (ACE2) activator diminazene aceturate ameliorates endotoxin-induced uveitis in mice. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3809–3818. [Google Scholar] [CrossRef] [PubMed]
- Foureaux, G.; Nogueira, J.C.; Nogueira, B.S.; Fulgêncio, G.O.; Menezes, G.B.; Fernandes, S.O.A.; Cardoso, V.N.; Fernandes, R.S.; Oliveira, G.P.; Franca, J.R.; et al. Antiglaucomatous effects of the activation of intrinsic angiotensin-converting enzyme 2. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4296–4306. [Google Scholar] [CrossRef]
- Neidhart, M.; Karouzakis, E.; Jüngel, A.; Gay, R.E.; Gay, S. Inhibition of spermidine/spermine N1-acetyltransferase activity: A new therapeutic concept in rheumatoid arthritis. Arthritis Rheumatol. 2014, 66, 1723–1733. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Macdonald, D.; Staunton, J.; Wilson, A.B. Methods and Compositions for the Treatment of Neurodegenerative. Disorders. Patent WO2008021210A2, 21 February 2008. Available online: https://patents.google.com/patent/WO2008021210A2 (accessed on 16 May 2019).
- Coma, M.; Aloy, P.; Pujol, A.; Gomis, X.; Oliva, B.; Lleó, A.; Mas, J.M. New Combination Therapies for Treating Neurological. Disorders. Patent WO2013061161A2, 2 May 2013. Available online: https://patents.google.com/patent/WO2013061161A2 (accessed on 16 May 2019).
- Ge, P.; Yao, X.; Li, J.; Jiang, R.; Dai, J.; Zhang, L. Diminazene aceturate alleviated lipopolysaccharide/D-galactosamine induced fulminant hepatitis in mice. Biomed. Pharmacother. 2018, 98, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.M.; Swan, G.E.; Lobetti, R.G.; Jacobson, L.S. The pharmacokinetics of diminazene aceturate after intramuscular administration in healthy dogs. J. S. Afr. Vet. Assoc. 2005, 76, 146–150. Available online: https://pdfs.semanticscholar.org/1b36/40339403b50a55bc5131872b2222e0dd8ab3.pdf (accessed on 16 May 2019). [CrossRef] [Green Version]
- Tiwari, G.; Tiwari, R.; Rai, A.K. Cyclodextrins in delivery systems: Applications. J. Pharm. Bioallied Sci. 2010, 2, 72–79. [Google Scholar] [CrossRef]
- Akode, R.M.; Shantier, S.W.; Gadkariem, E.A.; Mohamed, M.A. Simultaneous determination and stability studies on diminazene diaceturate and phenazone using developed derivative spectrophotometric method. Int. J. Anal. Chem. 2017, 2017. [Google Scholar] [CrossRef]
- Lupu, A.-C.; Barbacariu, A.; Roman, C.; Mîndru, R.; Martinescu, G.-V.; Cîmpan, A.-A.; Miron, L.D. In vivo study of conjugated diminazene aceturate for ichthyophthiriosis of farmed carp. Lucrări Științifice Medicină Veterinară 2018, 61, 65–75. [Google Scholar]
- Spulber, M.; Pinteala, M.; Harabagiu, V.; Simionescu, B.C. Inclusion complexes of sulconazole with beta-cyclodextrin and hydroxypropyl beta-cyclodextrin: Characterization in aqueous solution and in solid state. J. Incl. Phenom. Macrocycl. Chem. 2008, 61, 41–51. [Google Scholar] [CrossRef]
- Lewis, L.N.; Sumpter, C.A.; Sprenne, E.V.; Hedges, A.R.; Romberger, M.L. Purification of Cyclodextrin Complexes. U.S. Patent 5,403,828, 4 April 1995. [Google Scholar]
- Fielding, L. Determination of association constants (Ka) from solution NMR data. Tetrahedron 2000, 56, 6151–6170. [Google Scholar] [CrossRef]
- Malterud, K.E.; Rydland, K.M. Inhibitors of 15-lipoxygenase from orange peel. J. Agric. Food Chem. 2000, 48, 5576–5580. [Google Scholar] [CrossRef] [PubMed]
- Atsriku, C.; Watson, D.G.; Tettey, J.N.A.; Grant, M.H.; Skellern, G.G. Determination of diminazene aceturate in pharmaceutical formulations by HPLC and identification of related substances by LC/MS. J. Pharm. Biomed. Anal. 2002, 30, 979–986. [Google Scholar] [CrossRef]
- Koshiishi, I.; Mamura, Y.; Liu, J.; Imanari, T. Evaluation of an acidic deproteinization for the measurement of ascorbate and dehydroascorbate in plasma samples. Clin. Chem. 1998, 44, 863–868. [Google Scholar] [PubMed]
- Rossi, B.; Verrocchio, P.; Viliani, G.; Mancini, I.; Guella, G.; Rigo, E.; Scarduelli, G.; Mariotto, G. Vibrational properties of ibuprofen–cyclodextrin inclusion complexes investigated by Raman scattering and numerical simulation. J. Raman Spectrosc. 2009, 40, 453–458. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; John Wiley & sons, Ltd.: Chichester, UK, 2001. [Google Scholar]
- Iliescu, T.; Baia, M.; Miclăuş, V. A Raman spectroscopic study of the diclofenac sodium–β-cyclodextrin interaction. Eur. J. Pharm. Sci. 2004, 22, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Esme, A.; Sagdinc, S.G. The vibrational studies and theoretical investigation of structure, electronic and non-linear optical properties of Sudan III [1-{[4-(phenylazo) phenyl]azo}-2-naphthalenol]. J. Mol. Struct. 2013, 1048, 185–195. [Google Scholar] [CrossRef]
- Zimmermann, F.; Lippert, T.; Beyer, C.; Stebani, J.; Nuyken, O.; Wokaun, A. N=N Vibrational frequencies and fragmentation patterns of substituted 1-aryl-3,3-dialkyl-triazenes: Comparison with other high-nitrogen compounds. Appl. Spectrosc. 1993, 47, 986–993. [Google Scholar] [CrossRef]
- Mehenni, L.; Lahiani-Skiba, M.; Ladam, G.; Hallouard, F.; Skiba, M. Preparation and Characterization of spherical amorphous solid dispersion with amphotericin B. Pharmaceutics 2018, 10, 235. [Google Scholar] [CrossRef]
- Foureaux, G.; Franca, J.R.; Nogueira, J.C.; Fulgêncio Gde, O.; Ribeiro, T.G.; Castilho, R.O.; Yoshida, M.I.; Fuscaldi, L.L.; Fernandes, S.O.A.; Cardoso, V.N.; et al. Ocular inserts for sustained release of the angiotensin–converting enzyme 2 activator, diminazene aceturate, to treat glaucoma in rats. PLoS ONE 2015, 10, e0133149. [Google Scholar] [CrossRef]
- Minea, B.; Marangoci, N.; Peptanariu, D.; Rosca, I.; Nastasa, V.; Corciova, A.; Varganici, C.D.; Nicolescu, A.; Fifere, A.; Neamtu, A.; et al. Inclusion complexes of propiconazole nitrate with substituted β-cyclodextrins: The synthesis and in silico and in vitro assessment of their antifungal properties. New J. Chem. 2016, 40, 1765–1776. [Google Scholar] [CrossRef]
- Spulber, M.; Pinteala, M.; Fifere, A.; Harabagiu, V.; Simionescu, B.C. Inclusion complexes of 5-flucytosine with beta-cyclodextrin and hydroxypropyl-beta-cyclodextrin: Characterization in aqueous solution and in solid state. J. Incl. Phenom. Macrocycl. Chem. 2008, 62, 117–125. [Google Scholar] [CrossRef]
- Campbell, M.; Prankerd, R.J.; Davie, A.S.; Charman, W.N. Degradation of berenil (diminazene aceturate) in acidic aqueous solution. J. Pharm. Pharmacol. 2004, 56, 1327–1332. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Zhao, X.; Xu, Z.; Dong, N.; Zou, S.; Shen, X.; Huang, J. Development of a new colorimetric assay for lipoxygenase activity. Anal. Biochem. 2013, 441, 162–168. [Google Scholar] [CrossRef] [PubMed]














© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marangoci, N.; Timpu, D.; Corciova, A.; Mircea, C.; Petrovici, A.-R.; Nicolescu, A.; Ursu, E.-L.; Nastasa, V.; Bostanaru, A.-C.; Mares, M.; et al. β-Cyclodextrin as a Functional Excipient Used for Enhancing the Diminazene Aceturate Bioavailability. Pharmaceutics 2019, 11, 295. https://doi.org/10.3390/pharmaceutics11060295
Marangoci N, Timpu D, Corciova A, Mircea C, Petrovici A-R, Nicolescu A, Ursu E-L, Nastasa V, Bostanaru A-C, Mares M, et al. β-Cyclodextrin as a Functional Excipient Used for Enhancing the Diminazene Aceturate Bioavailability. Pharmaceutics. 2019; 11(6):295. https://doi.org/10.3390/pharmaceutics11060295
Chicago/Turabian StyleMarangoci, Narcisa, Daniel Timpu, Andreia Corciova, Cornelia Mircea, Anca-Roxana Petrovici, Alina Nicolescu, Elena-Laura Ursu, Valentin Nastasa, Andra-Cristina Bostanaru, Mihai Mares, and et al. 2019. "β-Cyclodextrin as a Functional Excipient Used for Enhancing the Diminazene Aceturate Bioavailability" Pharmaceutics 11, no. 6: 295. https://doi.org/10.3390/pharmaceutics11060295