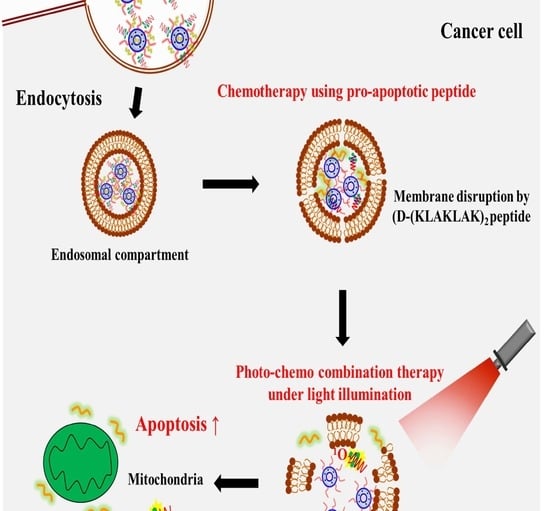
Co-delivery of D-(KLAKLAK)2 Peptide and Chlorin e6 using a Liposomal Complex for Synergistic Cancer Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Blank Liposome and Drug-Loaded Liposome
2.3. Measurement of Size and Zeta Potential of Liposomes
2.4. In Vitro Cellular Uptake Study
2.5. Caspase 3/7 Activity Measurement
2.6. Characterization of PEG-PLL(-g-Ce6)
2.7. Cytotoxicity
3. Results and Discussion
3.1. Preparation and Characterization of Liposomes
3.2. In Vitro Characterization of Lipo (Pep)
3.3. In Vitro Characterization of Lipo (Ce6)
3.4. In Vitro Evaluation of Lipo (Pep, Ce6)
3.5. Anticancer Activity of Lipo (Pep, Ce6)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Melo, F.D.S.E.; Vermeulen, L.; Fessler, E.; Medema, J.P. Cancer heterogeneity—A multifaceted view. EMBO Rep. 2013, 14, 686–695. [Google Scholar] [CrossRef] [PubMed]
- Persidis, A. Cancer multidrug resistance. Nat. Biotechnol. 1999, 17, 94. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C.; Reis-Filho, J.S. Genetic heterogeneity and cancer drug resistance. Lancet Oncol. 2012, 13, e178–e185. [Google Scholar] [CrossRef]
- Herskovic, A.; Martz, K.; Al-Sarraf, M.; Leichman, L.; Brindle, J.; Vaitkevicius, V.; Cooper, J.; Byhardt, R.; Davis, L.; Emami, B. Combined Chemotherapy and Radiotherapy Compared with Radiotherapy Alone in Patients with Cancer of the Esophagus. N. Engl. J. Med. 1992, 326, 1593–1598. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Han, J.; Shin, H.; Han, H.; Na, K.; Kim, H. Combination of chemotherapy and photodynamic therapy for cancer treatment with sonoporation effects. J. Control. Release 2018, 283, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, D.; Li, L.; Liu, T.; Tan, L.; Wu, X.; Tang, F. Multifunctional Gold Nanoshells on Silica Nanorattles: A Platform for the Combination of Photothermal Therapy and Chemotherapy with Low Systemic Toxicity. Angew. Chem. Int. Ed. 2011, 50, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Phung, D.C.; Nguyen, H.T.; Phuong Tran, T.T.; Jin, S.G.; Yong, C.S.; Truong, D.H.; Tran, T.H.; Kim, J.O. Combined hyperthermia and chemotherapy as a synergistic anticancer treatment. J. Pharm. Invest. 2019. [Google Scholar] [CrossRef]
- Dolmans, D.E.J.G.J.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380. [Google Scholar] [CrossRef]
- Hong, S.h.; Choi, Y. Mesoporous silica-based nanoplatforms for the delivery of photodynamic therapy agents. J. Pharm. Investig. 2018, 48, 3–17. [Google Scholar] [CrossRef]
- Hwang, H.S.; Shin, H.; Han, J.; Na, K. Combination of photodynamic therapy (PDT) and anti-tumor immunity in cancer therapy. J. Pharm. Investig. 2018, 48, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Jeon, G.; Ko, Y.T. Enhanced photodyamic therapy via photosensitizer-loaded nanoparticles for cancer treatment. J. Pharm. Invest. 2019, 49, 1–8. [Google Scholar] [CrossRef]
- Berg, K.; Kristian Selbo, P.; Prasmickaite, L.; Tjelle, T.E.; Sandvig, K.; Moan, J.; Gaudernack, G.; Fodstad, Ø.; Kjølsrud, S.; Anholt, H.; et al. Photochemical Internalization: A Novel Technology for Delivery of Macromolecules into Cytosol. Cancer Res. 1999, 59, 1180–1183. [Google Scholar] [PubMed]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part one—Photosensitizers, photochemistry and cellular localization. Photodiagn. Photodyn. Ther. 2004, 1, 279–293. [Google Scholar] [CrossRef]
- Choi, Y.H.; Han, H.-K. Nanomedicines: current status and future perspectives in aspect of drug delivery and pharmacokinetics. J. Pharm. Invest. 2018, 48, 43–60. [Google Scholar] [CrossRef] [PubMed]
- Ellerby, H.M.; Arap, W.; Ellerby, L.M.; Kain, R.; Andrusiak, R.; Rio, G.D.; Krajewski, S.; Lombardo, C.R.; Rao, R.; Ruoslahti, E.; et al. Anti-cancer activity of targeted pro-apoptotic peptides. Nat. Med. 1999, 5, 1032. [Google Scholar] [CrossRef] [PubMed]
- Mai, J.C.; Mi, Z.; Kim, S.-H.; Ng, B.; Robbins, P.D. A Proapoptotic Peptide for the Treatment of Solid Tumors. Cancer Res. 2001, 61, 7709–7712. [Google Scholar] [PubMed]
- Jiang, L.; Li, L.; He, X.; Yi, Q.; He, B.; Cao, J.; Pan, W.; Gu, Z. Overcoming drug-resistant lung cancer by paclitaxel loaded dual-functional liposomes with mitochondria targeting and pH-response. Biomaterials 2015, 52, 126–139. [Google Scholar] [CrossRef]
- Standley, S.M.; Toft, D.J.; Cheng, H.; Soukasene, S.; Chen, J.; Raja, S.M.; Band, V.; Band, H.; Cryns, V.L.; Stupp, S.I. Induction of Cancer Cell Death by Self-assembling Nanostructures Incorporating a Cytotoxic Peptide. Cancer Res. 2010, 70, 3020–3026. [Google Scholar] [CrossRef]
- Sun, J.; Jiang, L.; Lin, Y.; Gerhard, E.M.; Jiang, X.; Li, L.; Yang, J.; Gu, Z. Enhanced anticancer efficacy of paclitaxel through multistage tumor-targeting liposomes modified with RGD and KLA peptides. Int. J. Nanomed. 2017, 12, 1517–1537. [Google Scholar] [CrossRef]
- Johnson, G.A.; Muthukrishnan, N.; Pellois, J.-P. Photoinactivation of Gram Positive and Gram Negative Bacteria with the Antimicrobial Peptide (KLAKLAK)2 Conjugated to the Hydrophilic Photosensitizer Eosin Y. Bioconjug. Chem. 2013, 24, 114–123. [Google Scholar] [CrossRef]
- Han, S.-M.; Na, Y.-G.; Lee, H.-S.; Son, G.-H.; Jeon, S.-H.; Bang, K.-H.; Kim, S.-J.; Lee, H.-J.; Cho, C.-W. Improvement of cellular uptake of hydrophilic molecule, calcein, formulated by liposome. J. Pharm. Investig. 2018, 48, 595–601. [Google Scholar] [CrossRef]
- Danafar, H.; Rostamizadeh, K.; Hamidi, M. Polylactide/poly(ethylene glycol)/polylactide triblock copolymer micelles as carrier for delivery of hydrophilic and hydrophobic drugs: A comparison study. J. Pharm. Investig. 2018, 48, 381–391. [Google Scholar] [CrossRef]
- Au, G.H.T.; Shih, W.Y.; Shih, W.-H. Efficient intranuclear gene delivery by CdSe aqueous quantum dots electrostatically-coated with polyethyleneimine. Mater. Res. Express 2015, 2, 015401. [Google Scholar] [CrossRef]
- Ji, Y.; Zhao, J.; Chu, C.-C. Enhanced MHC-I antigen presentation from the delivery of ovalbumin by light-facilitated biodegradable poly(ester amide)s nanoparticles. J. Mater. Chem. B 2018, 6, 1930–1942. [Google Scholar] [CrossRef]
- Lim, C.; Moon, J.; Sim, T.; Won, W.R.; Lee, E.S.; Youn, Y.S.; Oh, K.T. A nano-complex system to overcome antagonistic photo-chemo combination cancer therapy. J. Control. Release 2019, 295, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Ku, E.B.; Oh, K.T.; Youn, Y.S.; Lee, E.S. Mitochondria-selective photodynamic tumor therapy using globular PEG nanoparticles. Macromol. Res. 2016, 24, 634–639. [Google Scholar] [CrossRef]
- Lee, J.M.; Kwag, D.S.; Youn, Y.S.; Lee, E.S. Gas-forming liposomes prepared using a liposomal magnetoporation method. Colloids Surf. B Biointerfaces 2017, 155, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Min, Y.; Bludau, H.; Keith, A.; Sheiko, S.S.; Jordan, R.; Wang, A.Z.; Sokolsky-Papkov, M.; Kabanov, A.V. Drug Combination Synergy in Worm-like Polymeric Micelles Improves Treatment Outcome for Small Cell and Non-Small Cell Lung Cancer. ACS Nano 2018, 12, 2426–2439. [Google Scholar] [CrossRef] [Green Version]
- Burns, K.E.; McCleerey, T.P.; Thévenin, D. pH-Selective Cytotoxicity of pHLIP-Antimicrobial Peptide Conjugates. Sci. Rep. 2016, 6, 28465. [Google Scholar] [CrossRef]
- Li, Y.; Jang, W.-D.; Nishiyama, N.; Kishimura, A.; Kawauchi, S.; Morimoto, Y.; Miake, S.; Yamashita, T.; Kikuchi, M.; Aida, T.; et al. Dendrimer Generation Effects on Photodynamic Efficacy of Dendrimer Porphyrins and Dendrimer-Loaded Supramolecular Nanocarriers. Chem. Mater. 2007, 19, 5557–5562. [Google Scholar] [CrossRef]
- Park, S.Y.; Baik, H.J.; Oh, Y.T.; Oh, K.T.; Youn, Y.S.; Lee, E.S. A Smart Polysaccharide/Drug Conjugate for Photodynamic Therapy. Angew. Chem. Int. Ed. 2011, 50, 1644–1647. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.; Sim, T.; Hoang, N.H.; Jung, C.E.; Lee, E.S.; Youn, Y.S.; Oh, K.T. A charge-reversible nanocarrier using PEG-PLL (-g-Ce6, DMA)-PLA for photodynamic therapy. Int. J. Nanomed. 2017, 12, 6185–6196. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Na, K. Conjugation of the photosensitizer Chlorin e6 to pluronic F127 for enhanced cellular internalization for photodynamic therapy. Biomaterials 2013, 34, 6992–7000. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, L.M.; Lorenzón, E.N.; Santos-Filho, N.A.; Zago, L.H.d.P.; Uliana, M.P.; de Oliveira, K.T.; Cilli, E.M.; Fontana, C.R. Antimicrobial Photodynamic therapy enhanced by the peptide aurein 1.2. Sci. Rep. 2018, 8, 4212. [Google Scholar] [CrossRef] [PubMed]
- Dariva, C.G.; Coelho, J.F.J.; Serra, A.C. Near infrared light-triggered nanoparticles using singlet oxygen photocleavage for drug delivery systems. J. Control. Release 2019, 294, 337–354. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.A.F.M.; Diebold, S.S.; Smyth, L.A.; Walters, A.A.; Lombardi, G.; Al-Jamal, K.T. Application of carbon nanotubes in cancer vaccines: Achievements, challenges and chances. J. Control. Release 2019, 297, 79–90. [Google Scholar] [CrossRef] [Green Version]
- Sato, Y.; Hashiba, K.; Sasaki, K.; Maeki, M.; Tokeshi, M.; Harashima, H. Understanding structure-activity relationships of pH-sensitive cationic lipids facilitates the rational identification of promising lipid nanoparticles for delivering siRNAs in vivo. J. Control. Release 2019, 295, 140–152. [Google Scholar] [CrossRef]






| Polymer | Size (nm) a | PDI a | Zeta potential (mV) a |
|---|---|---|---|
| Blank liposome | 118.7 ± 2.8 | 0.19 ± 0.02 | −10.6 ± 0.8 |
| Liposome/peptide (4:1) | 130.1 ± 3.2 | 0.22 ± 0.02 | −3.3 ± 0.9 |
| Liposome/peptide (8:1) | 111.6 ± 1.0 | 0.15 ± 0.03 | −4.1 ± 0.3 |
| Liposome/peptide (16:1) | 124.0 ± 0.6 | 0.19 ± 0.04 | −5.8 ± 0.4 |
| Liposome/peptide (32:1) | 126.2 ± 1.8 | 0.18 ± 0.02 | −4.7 ± 0.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, C.; Kang, J.K.; Won, W.R.; Park, J.Y.; Han, S.M.; Le, T.n.; Kim, J.C.; Her, J.; Shin, Y.; Oh, K.T. Co-delivery of D-(KLAKLAK)2 Peptide and Chlorin e6 using a Liposomal Complex for Synergistic Cancer Therapy. Pharmaceutics 2019, 11, 293. https://doi.org/10.3390/pharmaceutics11060293
Lim C, Kang JK, Won WR, Park JY, Han SM, Le Tn, Kim JC, Her J, Shin Y, Oh KT. Co-delivery of D-(KLAKLAK)2 Peptide and Chlorin e6 using a Liposomal Complex for Synergistic Cancer Therapy. Pharmaceutics. 2019; 11(6):293. https://doi.org/10.3390/pharmaceutics11060293
Chicago/Turabian StyleLim, Chaemin, Jin Kook Kang, Woong Roeck Won, June Yong Park, Sang Myung Han, Thi ngoc Le, Jae Chang Kim, Jaewon Her, Yuseon Shin, and Kyung Taek Oh. 2019. "Co-delivery of D-(KLAKLAK)2 Peptide and Chlorin e6 using a Liposomal Complex for Synergistic Cancer Therapy" Pharmaceutics 11, no. 6: 293. https://doi.org/10.3390/pharmaceutics11060293