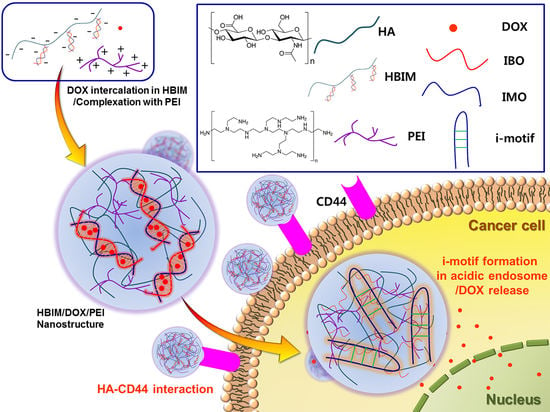
pH-Responsive i-motif Conjugated Hyaluronic Acid/Polyethylenimine Complexes for Drug Delivery Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Confirmation of ODN Hybridization and Its pH-Responsive Behavior
2.3. Interaction of ODN Hybrid with DOX
2.4. Synthesis of HA-IBO Conjugate (HB)
2.5. Confirmation of IBO Conjugation to HA
2.6. DOX Loading in HB-IMO hybrid (HBIM)
2.7. pH-Responsive Release of DOX
2.8. Particle Formation of HBIM with PEI1.8k (HBIM/PEI)
2.9. Cytotoxicity
2.10. Anticancer Efficacy of HBIM/DOX/PEI
3. Results and Discussion
3.1. Hybridization of IMO and IBO
3.2. Interaction of IBM with DOX
3.3. Synthesis and Characterization of HA-IBO Conjugate (HB)
3.4. DOX Loading and Release of HBIM
3.5. Particle Size and Zeta-Potential of HBIM/PEI
3.6. Cytotoxicity
3.7. Anticancer Efficacy of HBIM/DOX/PEI
3.8. Cellular Uptake of HBIM and HBIM/PEI Complexes in HeLa cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Leroy, J.L.; Guéron, M.; Mergny, J.L.; Hélène, C. Intramolecular folding of a fragment of the cytosine-rich strand of telomeric DNA into an I-motif. Nucleic Acids Res. 1994, 22, 1600–1606. [Google Scholar] [CrossRef] [PubMed]
- Guéron, M.; Leroy, J.L. The i-motif in nucleic acids. Curr. Opin. Struct. Biol. 2000, 10, 326–331. [Google Scholar] [CrossRef]
- Phan, A.T.; Mergny, J.-L. Human telomeric DNA: G-quadruplex, i-motif and Watson-Crick double helix. Nucleic Acids Res. 2002, 30, 4618–4625. [Google Scholar] [CrossRef] [PubMed]
- Benabou, S.; Aviñó, A.; Eritja, R.; González, C.; Gargallo, R. Fundamental aspects of the nucleic acid i-motif structures. RSC Adv. 2014, 4, 26956–26980. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Zhao, C.; Ren, J.; Qu, X. PH-controlled reversible drug binding and release using a cytosine-rich hairpin DNA. Chem. Commun. 2011, 47, 8043–8045. [Google Scholar] [CrossRef]
- Song, L.; Ho, V.H.B.; Chen, C.; Yang, Z.; Liu, D.; Chen, R.; Zhou, D. Efficient, pH-Triggered Drug Delivery Using a pH-Responsive DNA-Conjugated Gold Nanoparticle. Adv. Healthc. Mater. 2013, 2, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, Y.M.; Kang, Y.; Kim, W.J. Tumor-Homing, Size-Tunable Clustered Nanoparticles for Anticancer Therapeutics. ACS Nano 2014, 8, 9358–9367. [Google Scholar] [CrossRef]
- Park, H.; Kim, J.; Jung, S.; Kim, W.J. DNA-Au Nanomachine Equipped with i-Motif and G-Quadruplex for Triple Combinatorial Anti-Tumor Therapy. Adv. Funct. Mater. 2018, 28, 1705416. [Google Scholar] [CrossRef]
- Kim, J.Y.; Song, J.; Jung, H.; Mok, H. I-motif-coated exosomes as a pH-sensitive carrier for anticancer drugs. Appl. Biol. Chem. 2018, 61, 599–606. [Google Scholar] [CrossRef] [Green Version]
- Mero, A.; Campisi, M.; Mero, A.; Campisi, M. Hyaluronic Acid Bioconjugates for the Delivery of Bioactive Molecules. Polymers 2014, 6, 346–369. [Google Scholar] [CrossRef] [Green Version]
- Khaing, Z.Z.; Seidlits, S.K. Hyaluronic acid and neural stem cells: implications for biomaterial design. J. Mater. Chem. B 2015, 3, 7850–7866. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Moon, M.J.; Kim, D.Y.; Heo, S.H.; Jeong, Y.Y. Hyaluronic acid-based nanomaterials for cancer therapy. Polymers (Basel) 2018, 10, 1133. [Google Scholar] [CrossRef]
- Saravanakumar, G.; Deepagan, V.G.; Jayakumar, R.; Park, J.H. Hyaluronic acid-based conjugates for tumor-targeted drug delivery and imaging. J. Biomed. Nanotechnol. 2014, 10, 17–31. [Google Scholar] [CrossRef]
- Khunmanee, S.; Jeong, Y.; Park, H. Crosslinking method of hyaluronic-based hydrogel for biomedical applications. J. Tissue Eng. 2016, 8. [Google Scholar] [CrossRef]
- Tiwari, S.; Bahadur, P. Modified hyaluronic acid based materials for biomedical applications. Int. J. Biol. Macromol. 2019, 121, 556–571. [Google Scholar] [CrossRef]
- Olmsted, J.; Kearns, D.R. Mechanism of ethidium bromide fluorescence enhancement on binding to nucleic acids. Biochemistry 1977, 16, 3647–3654. [Google Scholar] [CrossRef]
- Mohan, P.; Rapoport, N. Doxorubicin as a Molecular Nanotheranostic Agent: Effect of Doxorubicin Encapsulation in Micelles or Nanoemulsions on the Ultrasound-Mediated Intracellular Delivery and Nuclear Trafficking. Mol. Pharm. 2010, 7, 1959–1973. [Google Scholar] [CrossRef] [Green Version]
- Rapoport, N.; Pitina, L. Intracellular Distribution and Intracellular Dynamics of a Spin-Labeled Analogue of Doxorubicin by Fluorescence and EPR Spectroscopy. J. Pharm. Sci. 1998, 87, 321–325. [Google Scholar] [CrossRef]
- Zhong, Y.; Goltsche, K.; Cheng, L.; Xie, F.; Meng, F.; Deng, C.; Zhong, Z.; Haag, R. Hyaluronic acid-shelled acid-activatable paclitaxel prodrug micelles effectively target and treat CD44-overexpressing human breast tumor xenografts in vivo. Biomaterials 2016, 84, 250–261. [Google Scholar] [CrossRef]
- Donghui, F.; Beibei, W.; Zheng, X.; Qisheng, G. Determination of hyaluronan by spectroscopic methods. J. Wuhan Univ. Technol. Sci. Ed. 2006, 21, 32–34. [Google Scholar] [CrossRef]
- Wu, Y. Preparation of low-molecular-weight hyaluronic acid by ozone treatment. Carbohydr. Polym. 2012, 89, 709–712. [Google Scholar] [CrossRef] [PubMed]
- Pershina, A.G.; Ogorodova, L.M.; Magaeva, A.A.; Itin, V.I.; Naiden, E.P.; Izaak, T.I.; Shchegoleva, N.N.; Sazonov, A.E. Sequence-selective binding of oligonucleotides to superparamagnetic cobalt ferrite nanoparticles: a new way to fabricate functional nanoconjugates. RSC Adv. 2015, 5, 26115–26124. [Google Scholar] [CrossRef]
- Volpi, N.; Maccari, F. Detection of submicrogram quantities of glycosaminoglycans on agarose gels by sequential staining with toluidine blue and Stains-All. Electrophoresis 2002, 23, 4060–4066. [Google Scholar] [CrossRef] [PubMed]
- Cong, W.-T.; Ye, W.-J.; Chen, M.; Zhao, T.; Zhu, Z.-X.; Niu, C.; Ruan, D.; Ni, M.-W.; Zhou, X.; Jin, L.-T. Improved staining of phosphoproteins with high sensitivity in polyacrylamide gels using Stains-All. Electrophoresis 2013, 34, 3277–3286. [Google Scholar] [CrossRef]
- Fröhlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef]
- Lehmann, T.; Köhler, C.; Weidauer, E.; Taege, C.; Foth, H. Expression of MRP1 and related transporters in human lung cells in culture. Toxicology 2001, 167, 59–72. [Google Scholar] [CrossRef]
- Salomon, J.J.; Ehrhardt, C. Nanoparticles attenuate P-glycoprotein/MDR1 function in A549 human alveolar epithelial cells. Eur. J. Pharm. Biopharm. 2011, 77, 392–397. [Google Scholar] [CrossRef]
- Kim, T.; Park, J.; Kim, T.-i. Cholic Acid-Conjugated Methylcellulose-Polyethylenimine Nano-Aggregates for Drug Delivery Systems. Nanomaterials 2019, 9, 459. [Google Scholar] [CrossRef]
- Huang, Y.; Cole, S.P.C.; Cai, T.; Cai, Y. Applications of nanoparticle drug delivery systems for the reversal of multidrug resistance in cancer. Oncol. Lett. 2016, 12, 11–15. [Google Scholar] [CrossRef]
- Yuan, Y.; Cai, T.; Xia, X.; Zhang, R.; Chiba, P.; Cai, Y. Nanoparticle delivery of anticancer drugs overcomes multidrug resistance in breast cancer. Drug Deliv. 2016, 23, 3350–3357. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Zeng, J.; Luo, L.; Yang, J.; Chen, J.; Li, B.; Shen, K. Identification of a cancer stem cell-like side population in the HeLa human cervical carcinoma cell line. Oncol. Lett. 2013, 6, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Mok, H.; Lee, S.; Oh, Y.-K.; Park, T.G. Target-specific intracellular delivery of siRNA using degradable hyaluronic acid nanogels. J. Control. Release 2007, 119, 245–252. [Google Scholar] [CrossRef] [PubMed]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, G.J.; Kim, T.-i. pH-Responsive i-motif Conjugated Hyaluronic Acid/Polyethylenimine Complexes for Drug Delivery Systems. Pharmaceutics 2019, 11, 247. https://doi.org/10.3390/pharmaceutics11050247
Lee GJ, Kim T-i. pH-Responsive i-motif Conjugated Hyaluronic Acid/Polyethylenimine Complexes for Drug Delivery Systems. Pharmaceutics. 2019; 11(5):247. https://doi.org/10.3390/pharmaceutics11050247
Chicago/Turabian StyleLee, Gyeong Jin, and Tae-il Kim. 2019. "pH-Responsive i-motif Conjugated Hyaluronic Acid/Polyethylenimine Complexes for Drug Delivery Systems" Pharmaceutics 11, no. 5: 247. https://doi.org/10.3390/pharmaceutics11050247