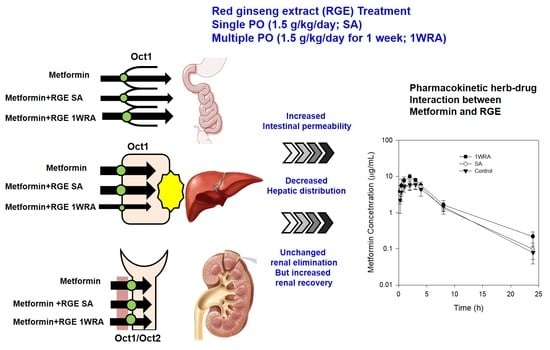
Enhanced Intestinal Permeability and Plasma Concentration of Metformin in Rats by the Repeated Administration of Red Ginseng Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Inhibitory effects of Red Ginseng Extract (RGE) on the Organic Cation Transport Activities
2.3. Animals and Ethical Approval
2.4. Analysis of Ginsenosides in RGE Extracts
2.5. Effect of RGE on mRNA and Protein Expression of Oct1 and Oct2 in the Intestine, Liver, and Kidney
2.6. Effect of RGE Treatment on the Permeability of Metformin
2.7. Effect of RGE on PK of Metformin in Rats
2.8. LC-MS/MS Analysis of Metformin
2.9. Data Analysis
3. Results
3.1. Concentration of Ginsenosides in RGE and Rat Plasma
3.2. Inhibitory Effects of RGE on the Transport Activities of OCT1, OCT2, MATE1, and MATE2-K
3.3. Effect of RGE on the Expression Level of Cationic Transporters
3.4. The Effect of RGE Treatment on the Intestinal Permeability of Metformin
3.5. Effect of RGE on the PK of metformin in rats
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Green, J.; Feinglos, M. New combination treatments in the management of diabetes: Focus on sitagliptin-metformin. Vasc. Health Risk Manag. 2008, 4, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Ahren, B. Novel combination treatment of type 2 diabetes dpp-4 inhibition + metformin. Vasc. Health Risk Manag. 2008, 4, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.K.; Jin, Q.R.; Ahn, S.H.; Bae, M.A.; Song, I.S. Sitagliptin attenuates metformin-mediated ampk phosphorylation through inhibition of organic cation transporters. Xenobiotica 2010, 40, 817–825. [Google Scholar] [CrossRef]
- Tucker, G.T.; Casey, C.; Phillips, P.J.; Connor, H.; Ward, J.D.; Woods, H.F. Metformin kinetics in healthy subjects and in patients with diabetes mellitus. Br. J. Clin. Pharmacol. 1981, 12, 235–246. [Google Scholar] [CrossRef]
- Proctor, W.R.; Ming, X.; Bourdet, D.; Han, T.K.; Everett, R.S.; Thakker, D.R. Why does the intestine lack basolateral efflux transporters for cationic compounds? A provocative hypothesis. J. Pharm. Sci. 2016, 105, 484–496. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.E.; Hong, S.S.; Choi, M.K.; Maeng, H.J.; Kim, D.D.; Chung, S.J.; Shim, C.K. Reduced antidiabetic effect of metformin and down-regulation of hepatic oct1 in rats with ethynylestradiol-induced cholestasis. Pharm. Res. 2009, 26, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Sheardown, S.A.; Brown, C.; Owen, R.P.; Zhang, S.; Castro, R.A.; Ianculescu, A.G.; Yue, L.; Lo, J.C.; Burchard, E.G.; et al. Effect of genetic variation in the organic cation transporter 1 (oct1) on metformin action. J. Clin. Investig. 2007, 117, 1422–1431. [Google Scholar] [CrossRef]
- Zang, M.; Zuccollo, A.; Hou, X.; Nagata, D.; Walsh, K.; Herscovitz, H.; Brecher, P.; Ruderman, N.B.; Cohen, R.A. Amp-activated protein kinase is required for the lipid-lowering effect of metformin in insulin-resistant human hepg2 cells. J. Biol. Chem. 2004, 279, 47898–47905. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of amp-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Goswami, S.; Giacomini, K.M.; Altman, R.B.; Klein, T.E. Metformin pathways: Pharmacokinetics and pharmacodynamics. Pharmacogenet. Genom. 2012, 22, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.; Choi, Y.A.; Choi, M.K.; Song, I.S. Organic cation transporter-mediated drug-drug interaction potential between berberine and metformin. Arch. Pharm. Res. 2015, 38, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, S.G.; Chae, I.G.; Kim, M.J.; Im, N.K.; Yu, M.H.; Lee, E.J.; Lee, I.S. Antioxidant effects of fermented red ginseng extracts in streptozotocin- induced diabetic rats. J. Ginseng Res. 2011, 35, 129–137. [Google Scholar] [CrossRef]
- Kim, J.H.; Pan, J.H.; Cho, H.T.; Kim, Y.J. Black ginseng extract counteracts streptozotocin-induced diabetes in mice. PLoS ONE 2016, 11, e0146843. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.J.; Han, Y.J.; Lee, W.; Kang, B.; Choi, M.K.; Han, Y.H.; Song, I.S. Effect of red ginseng extract on the pharmacokinetics and efficacy of metformin in streptozotocin-induced diabetic rats. Pharmaceutics 2018, 10, 80. [Google Scholar] [CrossRef]
- Oh, M.R.; Park, S.H.; Kim, S.Y.; Back, H.I.; Kim, M.G.; Jeon, J.Y.; Ha, K.C.; Na, W.T.; Cha, Y.S.; Park, B.H.; et al. Postprandial glucose-lowering effects of fermented red ginseng in subjects with impaired fasting glucose or type 2 diabetes: A randomized, double-blind, placebo-controlled clinical trial. BMC Complement. Altern Med. 2014, 14, 237. [Google Scholar] [CrossRef]
- Shishtar, E.; Sievenpiper, J.L.; Djedovic, V.; Cozma, A.I.; Ha, V.; Jayalath, V.H.; Jenkins, D.J.; Meija, S.B.; de Souza, R.J.; Jovanovski, E.; et al. The effect of ginseng (the genus panax) on glycemic control: A systematic review and meta-analysis of randomized controlled clinical trials. PLoS ONE 2014, 9, e107391. [Google Scholar] [CrossRef]
- Vuksan, V.; Sung, M.K.; Sievenpiper, J.L.; Stavro, P.M.; Jenkins, A.L.; Di Buono, M.; Lee, K.S.; Leiter, L.A.; Nam, K.Y.; Arnason, J.T.; et al. Korean red ginseng (Panax ginseng) improves glucose and insulin regulation in well-controlled, type 2 diabetes: Results of a randomized, double-blind, placebo-controlled study of efficacy and safety. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.J.; Kim, G.W.; Chung, S.H. Amp-activated protein kinase: An emerging target for ginseng. J. Ginseng Res. 2014, 38, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Kang, O.H.; Shon, M.Y.; Kong, R.; Seo, Y.S.; Zhou, T.; Kim, D.Y.; Kim, Y.S.; Kwon, D.Y. Anti-diabetic effect of black ginseng extract by augmentation of ampk protein activity and upregulation of glut2 and glut4 expression in db/db mice. BMC Complement. Altern. Med. 2017, 17, 341. [Google Scholar] [CrossRef] [PubMed]
- Cheon, J.M.; Kim, D.I.; Kim, K.S. Insulin sensitivity improvement of fermented Korean red ginseng (Panax ginseng) mediated by insulin resistance hallmarks in old-aged ob/ob mice. J. Ginseng Res. 2015, 39, 331–337. [Google Scholar] [CrossRef]
- Han, K.L.; Jung, M.H.; Sohn, J.H.; Hwang, J.K. Ginsenoside 20s-protopanaxatriol (ppt) activates peroxisome proliferator-activated receptor gamma (ppargamma) in 3t3-l1 adipocytes. Biol. Pharm. Bull. 2006, 29, 110–113. [Google Scholar] [CrossRef]
- Wu, Z.; Xie, Y.; Morrison, R.F.; Bucher, N.L.; Farmer, S.R. Ppargamma induces the insulin-dependent glucose transporter glut4 in the absence of c/ebpalpha during the conversion of 3t3 fibroblasts into adipocytes. J. Clin. Investig. 1998, 101, 22–32. [Google Scholar] [CrossRef]
- Yuan, H.D.; Kim, J.T.; Kim, S.H.; Chung, S.H. Ginseng and diabetes: The evidences from in vitro, animal and human studies. J. Ginseng Res. 2012, 36, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kwon, M.; Choi, M.K.; Song, I.S. Effects of red ginseng extract on the pharmacokinetics and elimination of methotrexate via mrp2 regulation. Molecules 2018, 23, 2948. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.N.; Ji, M.G.; Kang, T.H. The efficacy of red ginseng in type 1 and type 2 diabetes in animals. Evid. Based Complement. Alternat. Med. 2013, 2013, 593181. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.J.; Kim, N.; Lee, K.; Hee Sonn, C.; Eun Lee, J.; Tae Kim, S.; Ho Baeg, I.; Lee, K.M. Korean red ginseng (Panax ginseng) ameliorates type 1 diabetes and restores immune cell compartments. J. Ethnopharmacol. 2012, 144, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Hyun, H.B.; Lee, W.S.; Go, S.I.; Nagappan, A.; Park, C.; Han, M.H.; Hong, S.H.; Kim, G.; Kim, G.Y.; Cheong, J.; et al. The flavonoid morin from moraceae induces apoptosis by modulation of bcl-2 family members and fas receptor in hct 116 cells. Int. J. Oncol. 2015, 46, 2670–2678. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.U.; Kang, H.E.; Choi, S.Z.; Son, M.; Song, I.S.; Lee, H.S. Evaluation of drug--drug interaction potential between da-9801 and metformin. J. Pharm Investig. 2014, 44, 401–409. [Google Scholar] [CrossRef]
- Kang, H.J.; Song, I.J.; Lee, S.S.; Yoo, M.A.; Shin, J.G. Effects of dietary salt on the expression of drug transporters, cytochrome p4503a, and nuclear receptors in rats. Xenobiotica 2008, 38, 147–155. [Google Scholar] [CrossRef] [PubMed]
- You, B.H.; Chin, Y.W.; Kim, H.; Choi, H.S.; Choi, Y.H. Houttuynia cordata extract increased systemic exposure and liver concentrations of metformin through octs and mates in rats. Phytother. Res. 2018, 32, 1004–1013. [Google Scholar] [CrossRef]
- Kwon, M.; Ji, H.K.; Goo, S.H.; Nam, S.J.; Kang, Y.J.; Lee, E.; Liu, K.H.; Choi, M.K.; Song, I.S. Involvement of intestinal efflux and metabolic instability in the pharmacokinetics of platycodin d in rats. Drug Metab. Pharmacokinet. 2017, 32, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Gould, G.W.; Thomas, H.M.; Jess, T.J.; Bell, G.I. Expression of human glucose transporters in xenopus oocytes: Kinetic characterization and substrate specificities of the erythrocyte, liver, and brain isoforms. Biochemistry 1991, 30, 5139–5145. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Kusuhara, H.; Yokochi, M.; Toyoshima, J.; Inoue, K.; Yuasa, H.; Sugiyama, Y. Competitive inhibition of the luminal efflux by multidrug and toxin extrusions, but not basolateral uptake by organic cation transporter 2, is the likely mechanism underlying the pharmacokinetic drug-drug interactions caused by cimetidine in the kidney. J. Pharmacol. Exp. Ther. 2012, 340, 393–403. [Google Scholar] [CrossRef]
- Choi, M.K.; Song, I.S. Organic cation transporters and their pharmacokinetic and pharmacodynamic consequences. Drug Metab. Pharmacokinet. 2008, 23, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.A.; Yoon, Y.H.; Choi, K.; Kwon, M.; Goo, S.H.; Cha, J.S.; Choi, M.K.; Lee, H.S.; Song, I.S. Enhanced oral bioavailability of morin administered in mixed micelle formulation with pluronicf127 and tween80 in rats. Biol. Pharm. Bull. 2015, 38, 208–217. [Google Scholar] [CrossRef] [PubMed]
- International Transporter Consortium; Giacomini, K.M.; Huang, S.M.; Tweedie, D.J.; Benet, L.Z.; Brouwer, K.L.; Chu, X.; Dahlin, A.; Evers, R.; Fischer, V.; et al. Membrane transporters in drug development. Nat. Rev. Drug Discov. 2010, 9, 215–236. [Google Scholar] [PubMed]
- Park, S.E.; Na, C.S.; Yoo, S.A.; Seo, S.H.; Son, H.S. Biotransformation of major ginsenosides in ginsenoside model culture by lactic acid bacteria. J. Ginseng Res. 2017, 41, 36–42. [Google Scholar] [CrossRef]
- Wang, D.S.; Jonker, J.W.; Kato, Y.; Kusuhara, H.; Schinkel, A.H.; Sugiyama, Y. Involvement of organic cation transporter 1 in hepatic and intestinal distribution of metformin. J. Pharmacol. Exp. Ther. 2002, 302, 510–515. [Google Scholar] [CrossRef]
- Choi, Y.H.; Kim, S.G.; Lee, M.G. Dose-independent pharmacokinetics of metformin in rats: Hepatic and gastrointestinal first-pass effects. J. Pharm. Sci. 2006, 95, 2543–2552. [Google Scholar] [CrossRef]
- Nicklin, P.; Keates, A.C.; Page, T.; Bailey, C. Transfer of metformin across monolayes of human intestinal caco-2 cells and across rat intestine. Int. J. Pharm. 1996, 128, 155–162. [Google Scholar] [CrossRef]
- Choi, Y.H.; Lee, M.G. Effects of enzyme inducers and inhibitors on the pharmacokinetics of metformin in rats: Involvement of cyp2c11, 2d1 and 3a1/2 for the metabolism of metformin. Br. J. Pharmacol. 2006, 149, 424–430. [Google Scholar] [CrossRef]
- Jo, J.J.; Lee, S. Investigation of herb-drug interactions between korean red ginseng extract and five cyp substrates by lc-ms/ms. Mass Spectrom. Lett. 2017, 8, 98–104. [Google Scholar]
- Kim, H.; Nam, W.; Kim, S.H.; Jang, H.R.; Lee, M.K.; Kim, T.W.; Lee, S. Modulatory effects of korean red ginseng extract (Panax ginseng c.A. Meyer) on cytochrome p450 after oral administration to mice for 14 days. J. Life Sci. 2012, 22, 991–998. [Google Scholar] [CrossRef]
- Bogacz, A.; Karasiewicz, M.; Dziekan, K.; Procyk, D.; Górska-Paukszta, M.; Kowalska, A.; Mikołajczak, P.; Ożarowski, M.; Czerny, B. Impact of Panax ginseng and Ginkgo biloba extracts on expression level of transcriptional factors and xenobiotic-metabolizing cytochrome p450 enzymes. Herba Polonica 2016, 62, 42–54. [Google Scholar] [CrossRef]
- Maher, J.M.; Slitt, A.L.; Callaghan, T.N.; Cheng, X.; Cheung, C.; Gonzalez, F.J.; Klaassen, C.D. Alterations in transporter expression in liver, kidney, and duodenum after targeted disruption of the transcription factor hnf1alpha. Biochem. Pharmacol. 2006, 72, 512–522. [Google Scholar] [CrossRef]
- Lu, H.; Gonzalez, F.J.; Klaassen, C. Alterations in hepatic mrna expression of phase ii enzymes and xenobiotic transporters after targeted disruption of hepatocyte nuclear factor 4 alpha. Toxicol. Sci. 2010, 118, 380–390. [Google Scholar] [CrossRef]
- Maeda, T.; Oyabu, M.; Yotsumoto, T.; Higashi, R.; Nagata, K.; Yamazoe, Y.; Tamai, I. Effect of pregnane × receptor ligand on pharmacokinetics of substrates of organic cation transporter oct1 in rats. Drug Metab. Dispos. 2007, 35, 1580–1586. [Google Scholar] [CrossRef]
- O’Brien, V.P.; Bokelmann, K.; Ramirez, J.; Jobst, K.; Ratain, M.J.; Brockmoller, J.; Tzvetkov, M.V. Hepatocyte nuclear factor 1 regulates the expression of the organic cation transporter 1 via binding to an evolutionary conserved region in intron 1 of the oct1 gene. J. Pharmacol. Exp. Ther. 2013, 347, 181–192. [Google Scholar] [CrossRef]





| Ginsenosides | RGE | Rat Plasma | ||
|---|---|---|---|---|
| (μg/g RGE) | SA (ng/mL) | 1WRA (ng/mL) | ||
| 20(s)-protopanaxadiol | Rb1 | 2169.5 ± 312.1 | 14.3 ± 2.7 | 35.7 ± 1.9 |
| Rb2 | 856.3 ± 104.4 | 6.3 ± 2.1 | 29.4 ± 1.5 | |
| Rc | 1385.8 ± 164.0 | 9.0 ± 1.6 | 27.1 ± 1.3 | |
| Rd | 307.7 ± 48.4 | 2.9 ± 0.6 | 14.4 ± 2.6 | |
| Rh2 | ND | ND | ND | |
| Rg3 | 1534.9 ± 109.0 | ND | ND | |
| F2 | ND | ND | ND | |
| Compound K | ND | ND | ND | |
| Protopanaxadiol | ND | ND | ND | |
| 20(s)-protopanaxatriol | Re | 528.3 ± 78.9 | ND | ND |
| Rh1 | 490.7 ± 90.1 | ND | ND | |
| Rg1 | 999.4 ± 43.2 | ND | ND | |
| F1 | ND | ND | ND | |
| Protopanaxatriol | ND | ND | ND | |
| PK Parameters | Control | SA | 1WRA |
|---|---|---|---|
| T1/2 (h) | 1.10 ± 0.25 | 0.97 ± 0.16 | 1.32 ± 0.34 |
| C0 (ng/mL) | 1565.61 ± 247.60 | 1672.64 ± 623.06 | 1757.40 ± 437.84 |
| AUC24h (ng·h/mL) | 830.37 ± 211.80 | 1047.10 ± 236.94 | 980.12 ± 361.96 |
| AUC∞ (ng·h/mL) | 840.74 ± 212.54 | 1054.51 ± 239.22 | 985.62 ± 363.63 |
| MRT (h) | 0.70 ± 0.23 | 0.81 ± 0.17 | 0.85 ± 0.35 |
| Ae24h (%) | 80.07 ± 4.93 | 83.52 ± 5.72 | 79.56 ± 5.02 |
| PK Parameters | Control | SA | 1WRA |
|---|---|---|---|
| T1/2 (h) | 3.53 ± 0.74 | 3.56 ± 0.47 | 4.43 ± 0.74 * |
| Cmax (μg/mL) | 6.31 ± 1.68 | 6.47 ± 2.00 | 9.90 ± 1.49 * |
| Tmax (h) | 2.40 ± 0.89 | 2.50 ± 1.12 | 2.00 ± 0.01 |
| AUC24h (μg·h/mL) | 44.31 ± 8.31 | 45.20 ± 11.12 | 57.62 ± 4.99 * |
| AUC∞ (μg·h/mL) | 44.75 ± 8.09 | 45.72 ± 11.18 | 59.04 ± 5.17 * |
| MRT (h) | 4.85 ± 0.58 | 4.69 ± 0.33 | 4.64 ± 0.68 |
| Ae24h (%) | 54.56 ± 3.26 | 53.04 ± 3.69 | 66.29 ± 10.57 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, S.; Lee, S.; Jeon, J.-H.; Kim, H.; Choi, M.-K.; Song, I.-S. Enhanced Intestinal Permeability and Plasma Concentration of Metformin in Rats by the Repeated Administration of Red Ginseng Extract. Pharmaceutics 2019, 11, 189. https://doi.org/10.3390/pharmaceutics11040189
Jin S, Lee S, Jeon J-H, Kim H, Choi M-K, Song I-S. Enhanced Intestinal Permeability and Plasma Concentration of Metformin in Rats by the Repeated Administration of Red Ginseng Extract. Pharmaceutics. 2019; 11(4):189. https://doi.org/10.3390/pharmaceutics11040189
Chicago/Turabian StyleJin, Sojeong, Sowon Lee, Ji-Hyeon Jeon, Hyuna Kim, Min-Koo Choi, and Im-Sook Song. 2019. "Enhanced Intestinal Permeability and Plasma Concentration of Metformin in Rats by the Repeated Administration of Red Ginseng Extract" Pharmaceutics 11, no. 4: 189. https://doi.org/10.3390/pharmaceutics11040189