Investigating the Phospholipid Effect on the Bioaccessibility of Rosmarinic Acid-Phospholipid Complex through a Dynamic Gastrointestinal in Vitro Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the RA-PLC and Physical Mixture of RA and Phospholipid
2.3. Content Determination of RA in RA-PLC
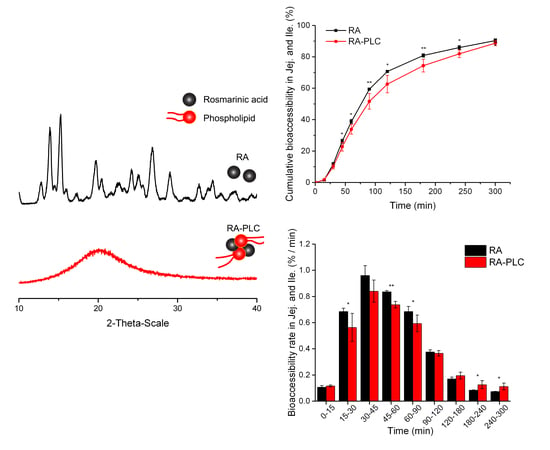
2.4. Powder X-ray Diffraction (PXRD)
2.5. Fourier-Transform Infrared (IR) Spectroscopy
2.6. n-Octanol/Water Partition Coefficient (P) Determination
2.7. Radical Scavenging Activity Assay
2.8. Cell Culture
2.9. Cell Viability Assay
2.10. Caco-2 Cell Transport Assay
2.11. TIM-1 Study
2.12. HPLC Analysis
2.13. Statistical Analysis
3. Results
3.1. RA Content in RA-PLC
3.2. PXRD Pattern
3.3. IR Spectroscopy
3.4. 1-Octanol/Water Partition Coefficient (P) of RA, RA–PLC, and PM
3.5. Radical Scavenging Activity (DPPH) Assay
3.6. Cell Viability
3.7. Caco-2 Transport Assay
3.8. In Vitro Bioaccessibility
3.8.1. Cumulative Bioaccessibility
3.8.2. Non-Cumulative Bioaccessibility
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bombardelli, E.; Curri, S.; DELLA LOGGIA, R.; Del Negro, P.; Gariboldi, P.; Tubaro, A. Complexes between phospholipids and vegetal derivates of biological interest. Fitoterapia 1989, 60, 1–9. [Google Scholar]
- Khan, J.; Alexander, A.; Ajazuddin; Saraf, S.; Saraf, S. Recent advances and future prospects of phyto-phospholipid complexation technique for improving pharmacokinetic profile of plant actives. J. Control. Release 2013, 168, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Semalty, A.; Semalty, M.; Singh, D.; Rawat, M. Development and characterization of aspirin-phospholipid complex for improved drug delivery. Int. J. Pharm. Sci. Nanotechnol. 2010, 3, 940–947. [Google Scholar]
- Mirza, S.; Miroshnyk, I.; Habib, M.J.; Brausch, J.F.; Hussain, M.D. Enhanced Dissolution and Oral Bioavailability of Piroxicam Formulations: Modulating Effect of Phospholipids. Pharmaceutics 2010, 2, 339–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jo, K.; Cho, J.M.; Lee, H.; Kim, E.K.; Kim, H.C.; Kim, H.; Lee, J. Enhancement of Aqueous Solubility and Dissolution of Celecoxib through Phosphatidylcholine-Based Dispersion Systems Solidified with Adsorbent Carriers. Pharmaceutics 2018, 11, 1. [Google Scholar] [CrossRef]
- Khazaeinia, T.; Jamali, F. A comparison of gastrointestinal permeability induced by diclofenac-phospholipid complex with diclofenac acid and its sodium salt. J. Pharm. Pharm. Sci. 2003, 6, 352–359. [Google Scholar] [PubMed]
- Peng, Q.; Zhang, Z.-R.; Sun, X.; Zuo, J.; Zhao, D.; Gong, T. Mechanisms of Phospholipid Complex Loaded Nanoparticles Enhancing the Oral Bioavailability. Mol. Pharm. 2010, 7, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Semalty, A.; Semalty, M.; Rawat, M.S.M.; Franceschi, F. Supramolecular phospholipids-polyphenolics interactions: The PHYTOSOME (R) strategy to improve the bioavailability of phytochemicals. Fitoterapia 2010, 81, 306–314. [Google Scholar] [CrossRef]
- Maiti, K.; Mukherjee, K.; Gantait, A.; Saha, B.P.; Mukherjee, P.K. Curcumin-phospholipid complex: Preparation, therapeutic evaluation and pharmacokinetic study in rats. Int. J. Pharm. 2007, 330, 155–163. [Google Scholar] [CrossRef]
- Yang, J.H.; Zhang, L.; Li, J.S.; Chen, L.H.; Zheng, Q.; Chen, T.; Chen, Z.P.; Fu, T.M.; Di, L.Q. Enhanced oral bioavailability and prophylactic effects on oxidative stress and hepatic damage of an oil solution containing a rosmarinic acid-phospholipid complex. J. Funct. Foods 2015, 19, 63–73. [Google Scholar] [CrossRef]
- Lichtenberger, L.M.; Wang, Z.M.; Romero, J.J.; Ulloa, C.; Perez, J.C.; Giraud, M.N.; Barreto, J.C. Non-steroidal anti-inflammatory drugs (NSAIDs) associate with zwitterionic phospholipids: Insight into the mechanism and reversal of NSAID-induced gastrointestinal injury. Nat. Med. 1995, 1, 154–158. [Google Scholar] [CrossRef]
- Belcaro, G.; Cesarone, M.; Dugall, M.; Pellegrini, L.; Ledda, A.; Grossi, M.; Togni, S.; Appendino, G. Product-evaluation registry of Meriva®, a curcumin-phosphatidylcholine complex, for the complementary management of osteoarthritis. Panminerva Med. 2010, 52 (Suppl. 1), 55–62. [Google Scholar] [PubMed]
- Kaplan, M.R.; Simoni, R.D. Intracellular transport of phosphatidylcholine to the plasma membrane. J. Cell Biol. 1985, 101, 441–445. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Saharan, V.A.; Singh, M.; Bhandari, A. Phytosome: Drug delivery system for polyphenolic phytoconstituents. Iran. J. Pharm. Sci. 2011, 7, 209–219. [Google Scholar]
- Kidd, P.; Head, K. A review of the bioavailability and clinical efficacy of milk thistle phytosome: A silybin-phosphatidylcholine complex (SiliphosR). Altern. Med. Rev. 2005, 10, 193–203. [Google Scholar]
- Li, Z.Y.; Agellon, L.B.; Allen, T.M.; Umeda, M.; Jewel, L.; Mason, A.; Vance, D.E. The ratio of phosphatidylcholine to phosphatidylethanolamine influences membrane integrity and steatohepatitis. Cell Metab. 2006, 3, 321–331. [Google Scholar] [CrossRef] [Green Version]
- Wirtz-Peitz, F.; Probst, M.; Winkelmann, J. Rosmarinic acid-phospholipide-complex. Google Patents 1982. [Google Scholar]
- Holst, B.; Williamson, G. Nutrients and phytochemicals: From bioavailability to bioefficacy beyond antioxidants. Curr. Opin. Biotechnol. 2008, 19, 73–82. [Google Scholar] [CrossRef]
- Chen, J.J.; Zheng, J.K.; Decker, E.A.; McClements, D.J.; Xiao, H. Improving nutraceutical bioavailability using mixed colloidal delivery systems: Lipid nanoparticles increase tangeretin bioaccessibility and absorption from tangeretin-loaded zein nanoparticles. RSC Adv. 2015, 5, 73892–73900. [Google Scholar] [CrossRef]
- Anson, N.M.; van den Berg, R.; Havenaar, R.; Bast, A.; Haenen, G. Bioavailability of ferulic acid is determined by its bioaccessibility. J. Cereal Sci. 2009, 49, 296–300. [Google Scholar] [CrossRef]
- Han, W.-L.; Lu, W.; He, D.-P.; Long, Y.-Q.; Shang, J.-C. Pharmacokinetics and Relative Bioavailability Study of Berberine Hydrochloride Phytosome in Rabbits. China Pharm. 2011, 17, 1564–1566. [Google Scholar]
- Jiang, Q.; Yang, X.; Du, P.; Zhang, H.; Zhang, T. Dual strategies to improve oral bioavailability of oleanolic acid: Enhancing water-solubility, permeability and inhibiting cytochrome P450 isozymes. Eur. J. Pharm. Biopharm. 2016, 99, 65–72. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y.; Deng, J.; Jia, X.; Zhou, J.; Lv, H. Solid dispersion of berberine-phospholipid complex/TPGS 1000/SiO2: Preparation, characterization and in vivo studies. Int. J. Pharm. 2014, 465, 306–316. [Google Scholar] [CrossRef]
- Weers, J.G.; Tarara, T.E.; Dellamary, L.A.; Riess, J.G.; Schutt, E.G. Phospholipid-Based Powders for Drug Delivery. U.S. Patent 7442388B2, 28 October 2008. [Google Scholar]
- Van Hoogevest, P.; Wendel, A. The use of natural and synthetic phospholipids as pharmaceutical excipients. Eur. J. Lipid Sci. Technol. 2014, 116, 1088–1107. [Google Scholar] [CrossRef] [PubMed]
- Van Hoogevest, P. Review—An update on the use of oral phospholipid excipients. Eur. J. Pharm. Sci. 2017, 108, 1–12. [Google Scholar] [CrossRef]
- Bermudez-Soto, M.J.; Tomas-Barberan, F.A.; Garcia-Conesa, M.T. Stability of polyphenols in chokeberry (Aronia melanocarpa) subjected to in vitro gastric and pancreatic digestion. Food Chem. 2007, 102, 865–874. [Google Scholar] [CrossRef]
- Wu, Z.; Teng, J.; Huang, L.; Xia, N.; Wei, B. Stability, antioxidant activity and in vitro bile acid-binding of green, black and dark tea polyphenols during simulated in vitro gastrointestinal digestion. RSC Adv. 2015, 5, 92089–92095. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Capanoglu, E.; Bilen, F.D.; Gonzales, G.B.; Grootaert, C.; Van de Wiele, T.; Van Camp, J. Bioaccessibility of Polyphenols from Plant-Processing Byproducts of Black Carrot (Daucus carota L.). J. Agric. Food Chem. 2016, 64, 2450–2458. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, L.; Kulisic-Bilusic, T.; Politeo, O.; Krause, I.; Dejanovic, B.; Ruberto, G. Phenolic Composition and Antioxidant Activity of Aqueous Infusions from Capparis spinosa L. and Crithmum maritimum L. before and after Submission to a Two-Step in Vitro Digestion Model. J. Agric. Food Chem. 2011, 59, 12453–12459. [Google Scholar] [CrossRef]
- Costa, P.; Grevenstuk, T.; Rosa da Costa, A.M.; Goncalves, S.; Romano, A. Antioxidant and anti-cholinesterase activities of Lavandula viridis L’Her extracts after in vitro gastrointestinal digestion. Ind. Crop. Prod. 2014, 55, 83–89. [Google Scholar] [CrossRef]
- Gayoso, L.; Claerbout, A.-S.; Isabel Calvo, M.; Yolanda Cavero, R.; Astiasaran, I.; Ansorena, D. Bioaccessibility of rutin, caffeic acid and rosmarinic acid: Influence of the in vitro gastrointestinal digestion models. J. Funct. Foods 2016, 26, 428–438. [Google Scholar] [CrossRef]
- Bhattacharya, S. Phytosomes: The new technology for enhancement of bioavailability of botanicals and nutraceuticals. Int. J. Health Res. 2009, 2, 225–232. [Google Scholar] [CrossRef]
- Blanquet, S.; Zeijdner, E.; Beyssac, E.; Meunier, J.P.; Denis, S.; Havenaar, R.; Alric, M. A dynamic artificial gastrointestinal system for studying the behavior of orally administered drug dosage forms under various physiological conditions. Pharm. Res. 2004, 21, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Lila, M.A.; Ribnicky, D.M.; Rojo, L.E.; Rojas-Silva, P.; Oren, A.; Havenaar, R.; Janle, E.M.; Raskin, I.; Yousef, G.G.; Grace, M.H. Complementary Approaches to Gauge the Bioavailability and Distribution of Ingested Berry Polyphenolics. J. Agric. Food Chem. 2012, 60, 5763–5771. [Google Scholar] [CrossRef] [PubMed]
- Verwei, M.; Arkbage, K.; Havenaar, R.; van den Berg, H.; Witthoft, C.; Schaafsma, G. Folic acid and 5-methyltetrahydrofolate in fortified milk are bioaccessible as determined in a dynamic in vitro gastrointestinal model. J. Nutr. 2003, 133, 2377–2383. [Google Scholar] [CrossRef] [PubMed]
- AlHasawi, F.M.; Fondaco, D.; Ben-Elazar, K.; Ben-Elazar, S.; Fan, Y.Y.; Corradini, M.G.; Ludescher, R.D.; Bolster, D.; Carder, G.; Chu, Y.; et al. In vitro measurements of luminal viscosity and glucose/maltose bioaccessibility for oat bran, instant oats, and steel cut oats. Food Hydrocoll. 2017, 70, 293–303. [Google Scholar] [CrossRef]
- Fondaco, D.; AlHasawi, F.; Lan, Y.; Ben-Elazar, S.; Connolly, K.; Rogers, M.A. Biophysical Aspects of Lipid Digestion in Human Breast Milk and SimilacTM Infant Formulas. Food Biophys. 2015, 10, 282–291. [Google Scholar] [CrossRef]
- Barker, R.; Abrahamsson, B.; Kruusmagi, M. Application and Validation of an Advanced Gastrointestinal In Vitro Model for the Evaluation of Drug Product Performance in Pharmaceutical Development. J. Pharm. Sci. 2014, 103, 3704–3712. [Google Scholar] [CrossRef]
- Kong, H.; Wang, M.; Venema, K.; Maathuis, A.; van der Heijden, R.; van der Greef, J.; Xu, G.; Hankemeier, T. Bioconversion of red ginseng saponins in the gastro-intestinal tract in vitro model studied by high-performance liquid chromatography-high resolution Fourier transform ion cyclotron resonance mass spectrometry. J. Chromatogr. A 2009, 1216, 2195–2203. [Google Scholar] [CrossRef] [PubMed]
- Verwei, M.; Minekus, M.; Zeijdner, E.; Schilderink, R.; Havenaar, R. Evaluation of two dynamic in vitro models simulating fasted and fed state conditions in the upper gastrointestinal tract (TIM-1 and tiny-TIM) for investigating the bioaccessibility of pharmaceutical compounds from oral dosage forms. Int. J. Pharm. 2016, 498, 178–186. [Google Scholar] [CrossRef]
- Butler, J.; Hens, B.; Vertzoni, M.; Brouwers, J.; Berben, P.; Dressman, J.; Andreas, C.J.; Schaefer, K.J.; Mann, J.; McAllister, M. In Vitro Models for the Prediction of in Vivo Performance of Oral Dosage Forms: Recent Progress from Partnership through the IMI OrBiTo Collaboration. Eur. J. Pharm. Biopharm. 2019, 136, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Van de Wiele, T.R.; Oomen, A.G.; Wragg, J.; Cave, M.; Minekus, M.; Hack, A.; Cornelis, C.; Rompelberg, C.J.; De Zwart, L.L.; Klinck, B. Comparison of Five in Vitro Digestion Models to in Vivo Experimental Results: Lead Bioaccessibility in the Human Gastrointestinal Tract. J. Environ. Sci. Health Part A 2007, 42, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Deng, Z.; Ramdath, D.D.; Tang, Y.; Chen, P.X.; Liu, R.; Liu, Q.; Tsao, R. Phenolic profiles of 20 Canadian lentil cultivars and their contribution to antioxidant activity and inhibitory effects on alpha-glucosidase and pancreatic lipase. Food Chem. 2015, 172, 862–872. [Google Scholar] [CrossRef]
- Minekus, M.; Marteau, P.; Havenaar, R.; Huisintveld, J.H.J. Multicompartmental dynamic computer-controlled model simulating the stomach and small intestine. ATLA 1995, 23, 197–209. [Google Scholar]
- Madureira, A.R.; Campos, D.A.; Fonte, P.; Nunes, S.; Reis, F.; Gomes, A.M.; Sarmento, B.; Pintado, M.M. Characterization of solid lipid nanoparticles produced with carnauba wax for rosmarinic acid oral delivery. RSC Adv. 2015, 5, 22665–22673. [Google Scholar] [CrossRef]
- Cevc, G. Phospholipids Handbook; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Boggs, J.M. Lipid intermolecular hydrogen bonding: influence on structural organization and membrane function. Biochim. Biophys. Acta 1987, 906, 353–404. [Google Scholar] [CrossRef]
- Eibl, H. The effect of the proton and of monovalent cations on membrane fluidity. Membr. Fluidity Biol. 1983, 2, 217. [Google Scholar]
- Marsh, D. Handbook of Lipid Bilayers; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Petersen, M.; Simmonds, M.S.J. Molecules of interest—Rosmarinic acid. Phytochemistry 2003, 62, 121–125. [Google Scholar] [CrossRef]
- Frankel, E.N.; Huang, S.W.; Aeschbach, R.; Prior, E. Antioxidant activity of a rosemary extract and its constituents, carnosic acid, carnosol, and rosmarinic acid, in bulk oil and oil-in-water emulsion. J. Agric. Food Chem. 1996, 44, 131–135. [Google Scholar] [CrossRef]
- Erkan, N.; Ayranci, G.; Ayranci, E. Antioxidant activities of rosemary (Rosmarinus Officinalis L.) extract, blackseed (Nigella sativa L.) essential oil, carnosic acid, rosmarinic acid and sesamol. Food Chem. 2008, 110, 76–82. [Google Scholar] [CrossRef]
- Chen, P.X.; Tang, Y.; Marcone, M.F.; Pauls, P.K.; Zhang, B.; Liu, R.H.; Tsao, R. Characterization of free, conjugated and bound phenolics and lipophilic antioxidants in regular- and non-darkening cranberry beans (Phaseolus vulgaris L.). Food Chem. 2015, 185, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Visconti, R.; Grieco, D. New insights on oxidative stress in cancer. Curr. Opin. Drug Discov. Dev. 2009, 12, 240–245. [Google Scholar]
- Artursson, P.; Karlsson, J. Correlation between Oral Drug Absorption in Humans and Apparent Drug Permeability Coefficients in Human Intestinal Epithelial (Caco-2) Cells. Biochem. Biophys. Res. Commun. 1991, 175, 880–885. [Google Scholar] [CrossRef]







| Sample | Media | Concentration in Original Aqueous Phase (C1) (μg/mL) | Concentration in Separated Aqueous Phase (C2) (μg/mL) | Partition Coefficient (C1 − C2)/C2 |
|---|---|---|---|---|
| RA | Millie Q water | 488 ± 8 | 139 ± 3 | 2.50 ± 0.06 |
| HCl (pH 1.2) | 500 ± 10 | 4.91 ± 0.09 | 100 ± 4 | |
| PBS (pH 6.8) | 483 ± 3 | 452 ± 4 | 0.068+0.005 | |
| PM | Millie Q water | 500 ± 4 | 136 ± 4 | 2.68 ± 0.09 |
| HCl (pH 1.2) | 350 ± 8 | 3.5 ± 0.3 | 100 ± 10 | |
| PBS (pH 6.8) | 491 ± 8 | 433 ± 6 | 0.131 ± 0.004 | |
| RA-PLC | Millie Q water | 500 ± 7 | 70 ± 3 | 6.2 ± 0.4 |
| HCl (pH 1.2) | 325 ± 7 | 3.25 ± 0.06 | 99 ± 3 | |
| PBS (pH 6.8) | 493 ± 6 | 423 ± 7 | 0.165 ± 0.005 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Chen, P.X.; Rogers, M.A.; Wettig, S.D. Investigating the Phospholipid Effect on the Bioaccessibility of Rosmarinic Acid-Phospholipid Complex through a Dynamic Gastrointestinal in Vitro Model. Pharmaceutics 2019, 11, 156. https://doi.org/10.3390/pharmaceutics11040156
Huang J, Chen PX, Rogers MA, Wettig SD. Investigating the Phospholipid Effect on the Bioaccessibility of Rosmarinic Acid-Phospholipid Complex through a Dynamic Gastrointestinal in Vitro Model. Pharmaceutics. 2019; 11(4):156. https://doi.org/10.3390/pharmaceutics11040156
Chicago/Turabian StyleHuang, Jiahao, Peter X. Chen, Michael A. Rogers, and Shawn D. Wettig. 2019. "Investigating the Phospholipid Effect on the Bioaccessibility of Rosmarinic Acid-Phospholipid Complex through a Dynamic Gastrointestinal in Vitro Model" Pharmaceutics 11, no. 4: 156. https://doi.org/10.3390/pharmaceutics11040156