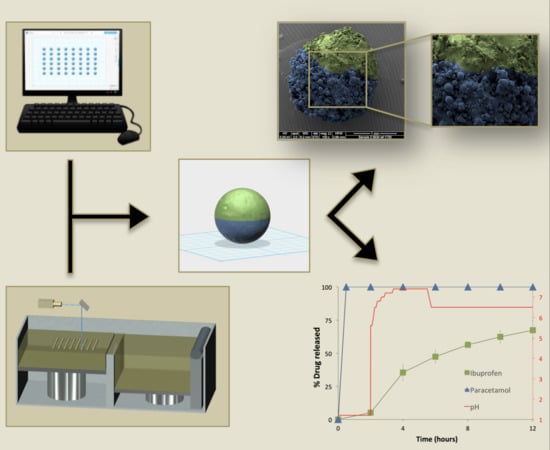
3D Printed Pellets (Miniprintlets): A Novel, Multi-Drug, Controlled Release Platform Technology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Printing Process
2.2. Thermal Analysis
2.3. X-ray Powder Diffraction (XRPD)
2.4. Characterisation of the Miniprintlets
2.4.1. Determination of the Miniprintlets Morphology
2.4.2. Scanning Electron Microscopy (SEM)
2.4.3. X-ray Micro Computed Tomography (Micro-CT)
2.4.4. Determination of Drug Content
2.5. In-Vitro Dissolution Testing
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Basit, A.W.; Gaisford, S. 3D Printing of Pharmaceuticals, 1st ed.; Springer International Publishing: Berlin, Germany, 2018. [Google Scholar] [CrossRef]
- Barnatt, C. 3D Printing, 3rd ed.; CreateSpace Independent Publishing Platform: Scotts Valley, SC, USA, 2016. [Google Scholar]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Arafat, B.; Qinna, N.; Cieszynska, M.; Forbes, R.T.; Alhnan, M.A. Tailored on demand anti-coagulant dosing: An in vitro and in vivo evaluation of 3D printed purpose-designed oral dosage forms. Eur. J. Pharm. Biopharm. 2018, 128, 282–289. [Google Scholar] [CrossRef]
- Verstraete, G.; Samaro, A.; Grymonpré, W.; Vanhoorne, V.; Van Snick, B.; Boone, M.N.; Hellemans, T.; Van Hoorebeke, L.; Remon, J.P.; Vervaet, C. 3D printing of high drug loaded dosage forms using thermoplastic polyurethanes. Int. J. Pharm. 2018, 536, 318–325. [Google Scholar] [CrossRef]
- Okwuosa, T.C.; Pereira, B.C.; Arafat, B.; Cieszynska, M.; Isreb, A.; Alhnan, M.A. Fabricating a shell-core delayed release tablet using dual fdm 3D printing for patient-centred therapy. Pharm. Res. 2017, 34, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Maroni, A.; Melocchi, A.; Parietti, F.; Foppoli, A.; Zema, L.; Gazzaniga, A. 3D printed multi-compartment capsular devices for two-pulse oral drug delivery. J. Control. Release 2017, 268, 10–18. [Google Scholar] [CrossRef]
- Fu, J.; Yu, X.; Jin, Y. 3D printing of vaginal rings with personalized shapes for controlled release of progesterone. Int. J. Pharm. 2018, 539, 75–82. [Google Scholar] [CrossRef]
- Goyanes, A.; Scarpa, M.; Kamlow, M.; Gaisford, S.; Basit, A.W.; Orlu, M. Patient acceptability of 3D printed medicines. Int. J. Pharm. 2017, 530, 71–78. [Google Scholar] [CrossRef]
- Arafat, B.; Wojsz, M.; Isreb, A.; Forbes, R.T.; Isreb, M.; Ahmed, W.; Arafat, T.; Alhnan, M.A. Tablet fragmentation without a disintegrant: A novel design approach for accelerating disintegration and drug release from 3D printed cellulosic tablets. Eur. J. Pharm. Sci. 2018, 118, 191–199. [Google Scholar] [CrossRef]
- Liang, K.; Carmone, S.; Brambilla, D.; Leroux, J.C. 3D printing of a wearable personalized oral delivery device: A first-in-human study. Sci. Adv. 2018, 4, eaat2544. [Google Scholar] [CrossRef] [Green Version]
- Melocchi, A.; Inverardi, N.; Uboldi, M.; Baldi, F.; Maroni, A.; Pandini, S.; Briatico-Vangosa, F.; Zema, L.; Gazzaniga, A. Retentive device for intravesical drug delivery based on water-induced shape memory response of poly(vinyl alcohol): Design concept and 4D printing feasibility. Int. J. Pharm. 2019. [Google Scholar] [CrossRef] [PubMed]
- Kollamaram, G.; Croker, D.M.; Walker, G.M.; Goyanes, A.; Basit, A.W.; Gaisford, S. Low temperature fused deposition modeling (FDM) 3D printing of thermolabile drugs. Int. J. Pharm. 2018, 545, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Kadry, H.; Al-Hilal, T.A.; Keshavarz, A.; Alam, F.; Xu, C.; Joy, A.; Ahsan, F. Multi-purposable filaments of hpmc for 3D printing of medications with tailored drug release and timed-absorption. Int. J. Pharm. 2018, 544, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Beck, R.C.R.; Chaves, P.S.; Goyanes, A.; Vukosavljevic, B.; Buanz, A.; Windbergs, M.; Basit, A.W.; Gaisford, S. 3D printed tablets loaded with polymeric nanocapsules: An innovative approach to produce customized drug delivery systems. Int. J. Pharm. 2017, 528, 268–279. [Google Scholar] [CrossRef]
- Janssen, R.B.I.; Moolenburgh, E.; Posthumus, B. TNO: The Impact of 3-D Printing on Supply Chain Management. Available online: http://3din.nl/wp-content/uploads/2014/02/TNO-Whitepaper-3-D-Printing-and-Supply-Chain-Management-April-2014-web.pdf (accessed on 2 March 2019).
- Awad, A.; Trenfield, S.J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Reshaping drug development using 3D printing. Drug Discov. Today 2018, 23, 1547–1555. [Google Scholar] [CrossRef] [PubMed]
- Kaae, S.; Lind, J.L.M.; Genina, N.; Sporrong, S.K. Unintended consequences for patients of future personalized pharmacoprinting. Int. J. Clin. Pharm. 2018. [Google Scholar] [CrossRef]
- Zema, L.; Melocchi, A.; Maroni, A.; Gazzaniga, A. Three-dimensional printing of medicinal products and the challenge of personalized therapy. J. Pharm. Sci. 2017, 106, 1697–1705. [Google Scholar] [CrossRef]
- Rowe, C.W.; Katstra, W.E.; Palazzolo, R.D.; Giritlioglu, B.; Teung, P.; Cima, M.J. Multimechanism oral dosage forms fabricated by three dimensional printing. J. Control. Release 2000, 66, 11–17. [Google Scholar] [CrossRef]
- Fina, F.; Gaisford, S.; Basit, A.W. Powder bed fusion: The working process, current applications and opportunities. In 3D Printing of Pharmaceuticals, 1st ed.; Basit, A.W., Gaisford, S., Eds.; Springer International Publishing: Berlin, Germany, 2018; pp. 81–105. [Google Scholar] [CrossRef]
- Eosoly, S.; Brabazon, D.; Lohfeld, S.; Looney, L. Selective laser sintering of hydroxyapatite/poly-ε-caprolactone scaffolds. Acta Biomaterialia 2010, 6, 2511–2517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trenfield, S.J.; Goyanes, A.; Telford, R.; Wilsdon, D.; Rowland, M.; Gaisford, S.; Basit, A.W. 3D printed drug products: Non-destructive dose verification using a rapid point-and-shoot approach. Int. J. Pharm. 2018, 549, 283–292. [Google Scholar] [CrossRef]
- Fina, F.; Madla, C.M.; Goyanes, A.; Zhang, J.; Gaisford, S.; Basit, A.W. Fabricating 3D printed orally disintegrating printlets using selective laser sintering. Int. J. Pharm. 2018, 541, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. Selective laser sintering (SLS) 3D printing of medicines. Int. J. Pharm. 2017, 529, 285–293. [Google Scholar] [CrossRef]
- Fina, F.; Goyanes, A.; Madla, C.M.; Awad, A.; Trenfield, S.J.; Kuek, J.M.; Patel, P.; Gaisford, S.; Basit, A.W. 3D printing of drug-loaded gyroid lattices using selective laser sintering. Int. J. Pharm. 2018, 547, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.M.; Adewunmi, A.; Schek, R.M.; Flanagan, C.L.; Krebsbach, P.H.; Feinberg, S.E.; Hollister, S.J.; Das, S. Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Biomaterials 2005, 26, 4817–4827. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Wang, M.; Zhou, W.Y.; Cheung, W.L.; Li, Z.Y.; Lu, W.W. Three-dimensional nanocomposite scaffolds fabricated via selective laser sintering for bone tissue engineering. Acta Biomaterialia 2010, 6, 4495–4505. [Google Scholar] [CrossRef]
- Ghebre-Selassie, I. Multiparticulate Oral Drug Delivery; Taylor & Francis: Oxford, UK, 1994. [Google Scholar]
- Davis, S.S.; Hardy, J.G.; Taylor, M.J.; Whalley, D.R.; Wilson, C.G. A comparative study of the gastrointestinal transit of a pellet and tablet formulation. Int. J. Pharm. 1984, 21, 167–177. [Google Scholar] [CrossRef]
- Goyanes, A.; Fernández-Ferreiro, A.; Majeed, A.; Gomez-Lado, N.; Awad, A.; Luaces-Rodríguez, A.; Gaisford, S.; Aguiar, P.; Basit, A.W. Pet/ct imaging of 3D printed devices in the gastrointestinal tract of rodents. Int. J. Pharm. 2018, 536, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; An, S.-H.; Rhee, Y.-S.; Park, C.-W.; Park, E.-S. A comparative study between spray-drying and fluidized bed coating processes for the preparation of pramipexole controlled-release microparticles for orally disintegrating tablets. Dry. Technol. 2014, 32, 935–945. [Google Scholar] [CrossRef]
- Yap, C.Y.; Chua, C.K.; Dong, Z.L.; Liu, Z.H.; Zhang, D.Q.; Loh, L.E.; Sing, S.L. Review of selective laser melting: Materials and applications. Appl. Phys. Rev. 2015, 2, 041101. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Merchant, H.A.; Kulkarni, R.P.; Alkademi, M.; Basit, A.W. Evolution of a physiological pH 6.8 bicarbonate buffer system: Application to the dissolution testing of enteric coated products. Eur. J. Pharm. Biopharm. 2011, 78, 151–157. [Google Scholar] [CrossRef]
- Fadda, H.M.; Basit, A.W. Dissolution of ph responsive formulations in media resembling intestinal fluids: Bicarbonate versus phosphate buffers. J. Drug Deliv. Sci. Technol. 2005, 15, 273–279. [Google Scholar] [CrossRef]
- Goyanes, A.; Hatton, G.B.; Basit, A.W. A dynamic in vitro model to evaluate the intestinal release behaviour of modified-release corticosteroid products. J. Drug Deliv. Sci. Technol. 2015, 25, 36–42. [Google Scholar] [CrossRef]
- Merchant, H.A.; Frost, J.; Basit, A.W. Apparatus and Method for Testing Medicaments. Patent PCT/GB2013/051145, 7 November 2013. [Google Scholar]
- Merchant, H.A.; Goyanes, A.; Parashar, N.; Basit, A.W. Predicting the gastrointestinal behaviour of modified-release products: Utility of a novel dynamic dissolution test apparatus involving the use of bicarbonate buffers. Int. J. Pharm. 2014, 475, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Moore, P.A.; Hersh, E.V. Combining ibuprofen and acetaminophen for acute pain management after third-molar extractions. J. Am. Dent. Assoc. 2013, 144, 898–908. [Google Scholar] [CrossRef] [PubMed]
- Mehlisch, D.R.; Aspley, S.; Daniels, S.E.; Bandy, D.P. Comparison of the analgesic efficacy of concurrent ibuprofen and paracetamol with ibuprofen or paracetamol alone in the management of moderate to severe acute postoperative dental pain in adolescents and adults: A randomized, double-blind, placebo-controlled, parallel-group, single-dose, two-center, modified factorial study. Clin. Ther. 2010, 32, 882–895. [Google Scholar] [CrossRef]
- Sibik, J.; Sargent, M.J.; Franklin, M.; Zeitler, J.A. Crystallization and phase changes in paracetamol from the amorphous solid to the liquid phase. Mol. Pharm. 2014, 11, 1326–1334. [Google Scholar] [CrossRef]
- Zhang, G.G.Z.; Paspal, S.Y.L.; Suryanarayanan, R.; Grant, D.J.W. Racemic species of sodium ibuprofen: Characterization and polymorphic relationships. J. Pharm. Sci. 2003, 92, 1356–1366. [Google Scholar] [CrossRef] [PubMed]
- Janssens, S.; Anné, M.; Rombaut, P.; Van den Mooter, G. Spray drying from complex solvent systems broadens the applicability of kollicoat IR as a carrier in the formulation of solid dispersions. Eur. J. Pharm. Sci. 2009, 37, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Hatton, G.B.; Merchant, H.A.; Basit, A.W. Gastrointestinal release behaviour of modified-release drug products: Dynamic dissolution testing of mesalazine formulations. Int. J. Pharm. 2015, 484, 103–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghebre-Sellassie, I.; Knoch, A. Pelletization techniques. In Encyclopedia of Pharmaceutical Technology; Swarbrick, J., Ed.; Informa Healthcare USA, Inc.: New York, NY, USA, 2007; Volume 3, pp. 2651–2663. [Google Scholar]
- Lee, H.; Lim, C.H.J.; Low, M.J.; Tham, N.; Murukeshan, V.M.; Kim, Y.-J. Lasers in additive manufacturing: A review. Int. J. Precis. Eng. Manuf.-Green Technol. 2017, 4, 307–322. [Google Scholar] [CrossRef]
- Neubert, V.; Czelusniak, T.; Lohrengel, A.F.; Higa, C.L.; Amorim, F. Selective laser sintering of mo-cuni composite to be used as edm electrode. Rapid Prototyp. J. 2014, 20, 59–68. [Google Scholar] [CrossRef]
- Goyanes, A.; Fina, F.; Martorana, A.; Sedough, D.; Gaisford, S.; Basit, A.W. Development of modified release 3D printed tablets (printlets) with pharmaceutical excipients using additive manufacturing. Int. J. Pharm. 2017, 527, 21–30. [Google Scholar] [CrossRef]
- Dukić-Ott, A.; Thommes, M.; Remon, J.P.; Kleinebudde, P.; Vervaet, C. Production of pellets via extrusion–spheronisation without the incorporation of microcrystalline cellulose: A critical review. Eur. J. Pharm. Biopharm. 2009, 71, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Trenfield, S.J.; Awad, A.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D printing pharmaceuticals: Drug development to frontline care. Trends Pharmacol. Sci. 2018, 39, 440–451. [Google Scholar] [CrossRef]
- Gioumouxouzis, C.I.; Baklavaridis, A.; Katsamenis, O.L.; Markopoulou, C.K.; Bouropoulos, N.; Tzetzis, D.; Fatouros, D.G. A 3D printed bilayer oral solid dosage form combining metformin for prolonged and glimepiride for immediate drug delivery. Eur. J. Pharm. Sci. 2018, 120, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.; Trenfield, S.J.; Gaisford, S.; Basit, A.W. 3D printed medicines: A new branch of digital healthcare. Int. J. Pharm. 2018, 548, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Palo, M.; Holländer, J.; Suominen, J.; Yliruusi, J.; Sandler, N. 3D printed drug delivery devices: Perspectives and technical challenges. Expert Rev. Med. Devices 2017, 14, 685–696. [Google Scholar] [CrossRef]
- Breitkreutz, J.; Boos, J. Paediatric and geriatric drug delivery. Expert Opin. Drug Deliv. 2007, 4, 37–45. [Google Scholar] [CrossRef]
- Martinez, P.R.; Goyanes, A.; Basit, A.W.; Gaisford, S. Influence of geometry on the drug release profiles of stereolithographic (SLA) 3D-printed tablets. AAPS PharmSciTech 2018, 19, 3355–3361. [Google Scholar] [CrossRef] [PubMed]
- Sadia, M.; Arafat, B.; Ahmed, W.; Forbes, R.T.; Alhnan, M.A. Channelled tablets: An innovative approach to accelerating drug release from 3D printed tablets. J. Control. Release 2018, 269, 355–363. [Google Scholar] [CrossRef]
- Vithani, K.; Goyanes, A.; Jannin, V.; Basit, A.W.; Gaisford, S.; Boyd, B.J. An overview of 3D printing technologies for soft materials and potential opportunities for lipid-based drug delivery systems. Pharm. Res. 2018, 36, 4. [Google Scholar] [CrossRef]








| Miniprintlets * | Paracetamol (Par) | Ibuprofen (Ibu) | Kollicoat Instant Release (KIR) | Ethyl Cellulose (EC) |
|---|---|---|---|---|
| Single | 5% | - | - | 92% |
| Dual–Con A | ||||
| Par/KIR | 6.5% | - | 56.5% | - |
| Ibu/EC | - | 3.5% | - | 30.5% |
| Dual–Con B | ||||
| Ibu/KIR | - | 3.5% | 30.5% | - |
| Par/EC | 6.5% | - | 56.5% |
| Miniprintlets | Weight (mg ± SD) | Diameter (mm ± SD) | Paracetamol Content (% ± SD) | Ibuprofen Content (% ± SD) |
|---|---|---|---|---|
| Single | ||||
| 1 mm | 0.84 ± 0.03 | 1.14 ± 0.06 | 101.1 ± 0.5 | - |
| 2 mm | 3.90 ± 0.13 | 1.99 ± 0.06 | 96.9 ± 0.2 | - |
| Dual–Con A | ||||
| 1 mm | 0.67 ± 0.03 | 1.04 ± 0.02 | 100.1 ± 1.5 * | 99.4 ± 1.0 * |
| 2 mm | 4.27 ± 0.15 | 2.03 ± 0.02 | 98.5 ± 1.5 * | 99.6 ± 1.4 * |
| Dual–Con B | ||||
| 1 mm | 0.50 ± 0.02 | 1.01 ± 0.04 | 99.2 ± 0.2 * | 98.3 ± 1.1 * |
| 2 mm | 4.10 ± 0.08 | 2.00 ± 0.03 | 96.6 ± 0.5 * | 100.2 ± 1.5 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awad, A.; Fina, F.; Trenfield, S.J.; Patel, P.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D Printed Pellets (Miniprintlets): A Novel, Multi-Drug, Controlled Release Platform Technology. Pharmaceutics 2019, 11, 148. https://doi.org/10.3390/pharmaceutics11040148
Awad A, Fina F, Trenfield SJ, Patel P, Goyanes A, Gaisford S, Basit AW. 3D Printed Pellets (Miniprintlets): A Novel, Multi-Drug, Controlled Release Platform Technology. Pharmaceutics. 2019; 11(4):148. https://doi.org/10.3390/pharmaceutics11040148
Chicago/Turabian StyleAwad, Atheer, Fabrizio Fina, Sarah J. Trenfield, Pavanesh Patel, Alvaro Goyanes, Simon Gaisford, and Abdul W. Basit. 2019. "3D Printed Pellets (Miniprintlets): A Novel, Multi-Drug, Controlled Release Platform Technology" Pharmaceutics 11, no. 4: 148. https://doi.org/10.3390/pharmaceutics11040148