Hyaluronic Acid-Modified and TPCA-1-Loaded Gold Nanocages Alleviate Inflammation
Abstract
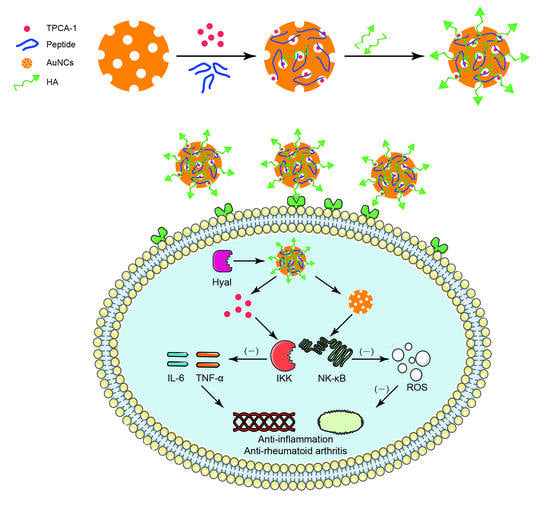
:1. Introduction
2. Materials and Methods
2.1. Reagents and Cell Lines
2.2. Synthesis of AuNCs
2.3. TPCA-1 Loading
2.4. Measurements and Characterizations
2.5. Cellular Uptake of HA-AuNCs/T/P
2.6. In Vitro Anti-Inflammatory Efficacy in RAW 264.7 Cells
2.7. Reactive Oxygen Species
2.8. Statistical Analysis
3. Results and Discussion
3.1. Synthesis and Characterization
3.2. In Vitro Study of HA-AuNCs/T/P
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, N.; Xu, C.Y.; Li, N.; Zhang, S.S.; Fu, L.L.; Chu, X.; Hua, H.Y.; Zeng, X.H.; Zhao, Y.X. Folate receptor-targeted mixed polysialic acid micelles for combating rheumatoid arthritis: In vitro and in vivo evaluation. Drug Deliv. 2018, 25, 1182–1191. [Google Scholar] [CrossRef]
- Brown, K.D.; Claudio, E.; Siebenlist, U. The roles of the classical and alternative nuclear factor-kappaB pathways: Potential implications for autoimmunity and rheumatoid arthritis. Arthritis Res. 2008, 10, 212. [Google Scholar] [CrossRef] [PubMed]
- Farahmand, L.; Darvishi, B.; Majidzadeh, A.K. Suppression of chronic inflammation with engineered nanomaterials delivering nuclear factor κB transcription factor decoy oligodeoxynucleotides. Drug Deliv. 2017, 24, 1249–1261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nan, J.; Du, Y.P.; Chen, X.; Bai, Q.; Wang, Y.; Zhang, X.X.; Zhu, N.; Zhang, J.; Hou, J.W.; Wang, Q.; et al. TPCA-1 is a direct dual inhibitor of STAT3 and NF-kappaB and regresses mutant EGFR-associated human non-small cell lung cancers. Mol. Cancer 2014, 13, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Gaddipati, S.; Lu, Q.; Kasetti, R.B.; Miller, M.C.; Lu, Q.; Trent, J.O.; Kaplan, H.J.; Li, Q. IKK2 inhibition using TPCA-1-loaded PLGA microparticles attenuates laser-induced chor, oidal neovascularization and macrophage recruitment. PLoS ONE 2015, 10, e0121185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wardwell, P.R.; Bader, R.A. Polysaccharide-based micelles for drug delivery. Pharmaceutics 2013, 5, 329–352. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Z.; Chen, Z.W.; Liu, Z.; Shi, P.; Dong, K.; Ju, E.G.; Ren, J.S.; Qu, X.J. A multi-stimuli responsive gold nanocage-hyaluronic platform for targeted photothermal and chemotherapy. Biomaterials 2014, 35, 9678–9688. [Google Scholar] [CrossRef] [PubMed]
- Parashar, P.; Rathor, M.; Dwivedi, M.; Saraf, S.A. Hyaluronic Acid Decorated Naringenin Nanoparticles: Appraisal of Chemopreventive and Curative Potential for Lung Cancer. Pharmaceutics 2018, 10, 33. [Google Scholar] [CrossRef]
- Bao, S.; Huang, S.; Liu, Y.; Hu, Y.; Wang, W.; Ji, M.; Li, H.; Zhang, N.X.; Song, C.; Duan, S. Gold nanocages with dual modality for image-guided therapeutics. Nanoscale 2017, 9, 7284–7296. [Google Scholar] [CrossRef]
- Gao, L.; Fei, J.; Zhao, J.; Li, H.; Cui, Y.; Li, J. Hypocrellin-loaded gold nanocages with high two-photon efficiency for photothermal/photodynamic cancer therapy in vitro. ACS Nano 2012, 6, 8030–8040. [Google Scholar] [CrossRef]
- Huang, S.N.; Ying, L.; Xin, X.; Ji, M.F.; Li, Y.M.; Song, C.J.; Duan, S.F.; Hu, Y.R. Triple therapy of hepatocellular carcinoma with microRNA-122 and doxorubicin co-loaded functionalized gold nanocages. J. Mater. Chem. B 2018, 6, 2217–2229. [Google Scholar] [CrossRef]
- Thakor, A.S.; Jokerst, J.; Zavaleta, C.; Massoud, T.F.; Gambhir, S.S. Gold nanoparticles: A revival in precious metal administration to patients. Nano Lett. 2011, 11, 4029–4036. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, M.Y.; Bhang, S.H.; Kim, B.S.; Kim, Y.S.; Ju, J.H.; Kim, K.S.; Hahn, S.K. Hyaluronate-gold nanoparticle/tocilizumab complex for the treatment of rheumatoid arthritis. ACS Nano 2014, 8, 4790–4798. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Kim, H.J.; Ha, Y.J.; Park, Y.N.; Lee, S.K.; Park, Y.B.; Yoo, K.H. Targeted chemo-photothermal treatments of rheumatoid arthritis using gold half-shell multifunctional nanoparticles. ACS Nano 2013, 7, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Guo, Z.; Jiang, X.; Fang, K.; Lu, X.; Zhang, Y.; Gu, N. Quasi-spherical silver nanoparticles: Aqueous synthesis and size control by the seed-mediated Lee-Meisel method. J. Colloid Interface Sci. 2013, 394, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Podolin., P.L.; Callahan, J.F.; Bolognese, B.J.; Li, Y.H.; Carlson, K.; Davis, T.G.; Mellor, G.W.; Evans, C.; Roshak, A.K. Attenuation of murine collagen-induced arthritis by a novel, potent, selective small molecule inhibitor of IkappaB Kinase 2,TPCA-1(2-[(aminocarbonyl)amino]-5-(4-fluorophenyl)-3-thiophenecarboxamide), occurs via reduction of proinflammatory cytokines and antigen-induced T cell Proliferation. J. Pharmacol. Exp. Ther. 2005, 312, 373–381. [Google Scholar] [PubMed]
- Hua, H.; Zhang, N.; Liu, D.; Song, L.; Liu, T.; Li, S.; Zhao, Y. Multifunctional gold nanorods and docetaxel-encapsulated liposomes for combined thermo- and chemotherapy. Int. J. Nanomed. 2017, 12, 7869–7884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, A.; Saltik, M.; Lehrmann, H.; Killisch, I.; Mautner, V.; Lamm, G.; Christofori, G.; Cotten, M. Polyethylenimine (PEI) is a simple, inexpensive and effective reagent for condensing and linking plasmid DNA to adenovirus for gene delivery. Gene. Ther. 1997, 4, 773–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, N.; Tao, J.; Hua, H.; Sun, P.; Zhao, Y. Low-density lipoprotein peptide-combined DNA nanocomplex as an efficient anticancer drug delivery vehicle. Eur. J. Pharm. Biopharm. 2015, 94, 20–29. [Google Scholar] [CrossRef]
- Yoshimura, S.; Bondeson, J.; Brennan, F.M.; Foxwell, B.M.; Feldmann, M. Antigen presentation by murine dendritic cells is nuclear factor-kappa B dependent both in vitro and in vivo. Scand. J. Immunol. 2003, 5, 165–172. [Google Scholar] [CrossRef]
- Boffa, D.J.; Feng, B.; Sharma, V.; Dematteo, R.; Miller, G.; Suthanthiran, M.; Nunez, R.; Liou, H.C. Selective loss of c-Rel compromises dendritic cell activation of T lymphocytes. Cell Immunol. 2003, 222, 105–115. [Google Scholar] [CrossRef]
- Sul, O.J.; Kim, J.C.; Kyung, T.W.; Kim, H.J.; Kim, Y.Y.; Kim, S.H.; Kim, J.S.; Choi, H.S. Gold nanoparticles inhibited the receptor activator of nuclear factor-κb ligand (RANKL)-induced osteoclast formation by acting as an antioxidant. Biosci. Biotechnol. Biochem. 2010, 74, 2209–2213. [Google Scholar] [CrossRef] [PubMed]
- Mackey, M.A.; Saira, F.; Mahmoud, M.A.; El-Sayed, M.A. Inducing cancer cell death by targeting its nucleus: Solid gold nanospheres versus hollow gold nanocages. Bioconjug. Chem. 2013, 24, 897–906. [Google Scholar] [CrossRef] [PubMed]




© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J. Hyaluronic Acid-Modified and TPCA-1-Loaded Gold Nanocages Alleviate Inflammation. Pharmaceutics 2019, 11, 143. https://doi.org/10.3390/pharmaceutics11030143
Zhao J. Hyaluronic Acid-Modified and TPCA-1-Loaded Gold Nanocages Alleviate Inflammation. Pharmaceutics. 2019; 11(3):143. https://doi.org/10.3390/pharmaceutics11030143
Chicago/Turabian StyleZhao, Jingnan. 2019. "Hyaluronic Acid-Modified and TPCA-1-Loaded Gold Nanocages Alleviate Inflammation" Pharmaceutics 11, no. 3: 143. https://doi.org/10.3390/pharmaceutics11030143