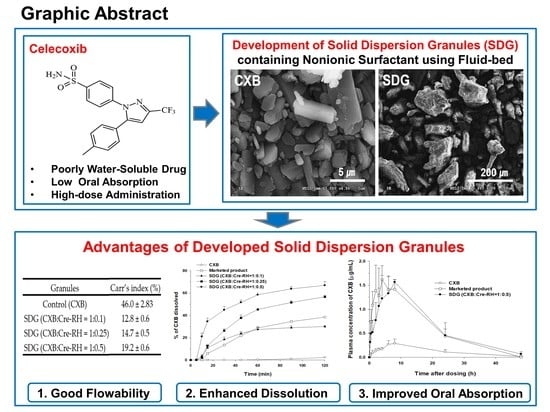
Development and Evaluation of Poorly Water-Soluble Celecoxib as Solid Dispersions Containing Nonionic Surfactants Using Fluidized-Bed Granulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Determination of CXB Solubility in Solution with Solubilizer
2.3. Preparation of Solid Dispersion and Tablets
2.4. Scanning Electron Microscopy (SEM)
2.5. Differential Scanning Calorimetry (DSC)
2.6. Flowability of SDG
2.7. Hardness and Friability of SDG Tablet
2.8. Dissolution Study of SDGs and SDG Tablets
2.9. In Vivo Oral Pharmacokinetic Studies in Rats
3. Results
3.1. Selection of an Optimal Solubilizer for Solid Dispersion System
3.2. Physicochemical Characterizations of Solid Dispersion Granule and Tablet
3.2.1. Formulation and Morphology
3.2.2. Solid-State Characterization
3.2.3. Dissolution of CXB from SDG and SDT
3.3. In Vivo Oral Pharmacokinetic Studies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vasconcelos, T.; Sarmento, B.; Costa, P. Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs. Drug Discov. Today 2007, 12, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Heo, M.Y.; Piao, Z.Z.; Kim, T.W.; Cao, Q.R.; Kim, A.; Lee, B.J. Effect of solubilizing and microemulsifying excipients in polyethylene glycol 6000 solid dispersion on enhanced dissolution and bioavailability of ketoconazole. Arch. Pharmacal Res. 2005, 28, 604–611. [Google Scholar] [CrossRef]
- Song, I.S.; Cha, J.S.; Choi, M.K. Characterization, in vivo and in vitro evaluation of solid dispersion of curcumin containing d-α-tocopheryl polyethylene glycol 1000 succinate and mannitol. Molecules 2016, 21, 1386. [Google Scholar] [CrossRef] [PubMed]
- Craig, D.Q.M. The mechanisms of drug release from solid dispersions in water-soluble polymers. Int. J. Pharm. 2002, 231, 131–144. [Google Scholar] [CrossRef]
- Ho, H.O.; Su, H.L.; Tsai, T.; Sheu, M.T. The preparation and characterization of solid dispersions on pellets using a fluidized-bed system. Int. J. Pharm. 1996, 139, 223–229. [Google Scholar] [CrossRef]
- Gohel, M.C.; Patel, L.D. Processing of nimesulide-PEG 400-PG-PVP solid dispersions: Preparation, characterization, and in vitro dissolution. Drug Dev. Ind. Pharm. 2003, 29, 299–310. [Google Scholar] [CrossRef]
- Sun, N.; Wei, X.; Wu, B.; Chen, J.; Lu, Y.; Wu, W. Enhanced dissolution of silymarin/polyvinylpyrrolidone solid dispersion pellets prepared by a one-step fluid-bed coating technique. Powder Technol. 2008, 182, 72–80. [Google Scholar] [CrossRef]
- Serajuddin, A.T. Solid dispersion of poorly water-soluble drugs: Early promises, subsequent problems, and recent breakthroughs. J. Pharm. Sci. 2000, 88, 1058–1066. [Google Scholar] [CrossRef]
- Rajniak, P.; Stepanek, F.; Dhanasekharan, K.; Fan, R.; Mancinelli, C.; Chern, R.T. A combined experimental and computational study of wet granulation in a Wurster fluid bed granulator. Powder Technol. 2009, 189, 190–201. [Google Scholar] [CrossRef]
- Vercruysse, J.; Díaz, D.C.; Peeters, E.; Fonteyne, M.; Delaet, U.; van Assche, I.; de Beer, T.; Remon, J.P.; Vervaet, C. Continuous twin screw granulation: Influence of process variables on granule and tablet quality. Eur. J. Pharm. Biopharm. 2012, 82, 205–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burggraeve, A.; Monteyne, T.; Vervaet, C.; Remon, J.P.; de Beer, T. Process analytical tools for monitoring, understanding, and control of pharmaceutical fluidized bed granulation: A review. Eur. J. Pharm. Biopharm. 2013, 83, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Clemett, D.; Goa, K.L. Celecoxib. Drugs 2000, 59, 957–980. [Google Scholar] [CrossRef] [PubMed]
- Ryn, J.; Trummlitz, G.; Pairet, M. COX-2 Selectivity and Inflammatory Processes. Curr. Med. Chem. 2000, 7, 1145–1161. [Google Scholar]
- Dougados, M.; Béhier, J.M.; Jolchine, I.; Calin, A.; van der Heijde, D.; Olivieri, I.; Zeidler, H.; Herman, H. Efficacy of Celecoxib, a Cyclooxygenase 2–Specific Inhibitor, in the Treatment of Ankylosing Spondylitis. Arthritis Rheumatol. 2001, 44, 180–185. [Google Scholar] [CrossRef]
- Paulson, S.K.; Vaughn, M.B.; Jessen, S.M.; Lawal, Y.; Gresk, C.J.; Yan, B.; Maziasz, T.J.; Cook, C.S.; Karim, A. Pharmacokinetics of celecoxib after oral administration in dogs and humans: Effect of food and site of absorption. J. Pharmacol. Exp. Ther. 2001, 297, 638–645. [Google Scholar]
- Shi, S.; Klotz, U. Clinical use and pharmacological properties of selective COX-2 inhibitors. Eur. J. Clin. Pharmacol. 2008, 64, 223–252. [Google Scholar] [CrossRef] [PubMed]
- Lainé, A.L.; Price, D.; Davis, J.; Roberts, D.; Hudson, R.; Back, K.; Bungay, P.; Flanagan, N. Enhanced oral delivery of celecoxib via the development of a supersaturable amorphous formulation utilising mesoporous silica and co-loaded HPMCAS. Int. J. Pharm. 2016, 512, 118–125. [Google Scholar] [CrossRef]
- Van Nguyen, H.; Park, C.; Oh, E.; Lee, B.J. Improving the dissolution rate of a poorly water-soluble drug via adsorption onto pharmaceutical diluents. J. Drug Deliv. Sci. Technol. 2016, 35, 146–154. [Google Scholar] [CrossRef]
- De Jongh, C.M.; Verberk, M.M.; Withagen, C.E.; Jacobs, J.J.; Rustemeyer, T.; Kezic, S. Stratum corneum cytokines and skin irritation response to sodium lauryl sulfate. Contact Dermat. 2006, 54, 325–333. [Google Scholar] [CrossRef]
- Kosswig, K. Surfactants. In Ullmann’s Encyclopedia of Industrial Chemistry, 5th ed.; Wiley-VCH Verlag: Weinheim, Germany, 2000; p. 495. ISBN 9783527303854. [Google Scholar]
- Cserháti, T.; Forgács, E.; Oros, G. Biological activity and environmental impact of anionic surfactants. Environ. Int. 2002, 28, 337–348. [Google Scholar] [CrossRef]
- Ghosh, I.; Bose, S.; Vippagunta, R.; Harmon, F. Nanosuspension for improving the bioavailability of a poorly soluble drug and screening of stabilizing agents to inhibit crystal growth. Int. J. Pharm. 2011, 409, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.; Arteaga, C.; Abdelmalek, B.; Davé, R.; Bilgili, E. Spray drying of drug-swellable dispersant suspensions for preparation of fast-dissolving, high drug-loaded, surfactant-free nanocomposites. Drug Dev. Ind. Pharm. 2015, 41, 1617–1631. [Google Scholar] [CrossRef] [PubMed]
- Edwards, D.A.; Luthy, R.G.; Liu, Z. Solubilization of polycyclic aromatic hydrocarbons in micellar nonionic surfactant solutions. Environ. Sci. Technol. 1991, 25, 127–133. [Google Scholar] [CrossRef]
- Kim, H.I.; Park, S.Y.; Park, S.J.; Lee, J.W.; Cho, K.H.; Jee, J.P.; Kim, H.C.; Maeng, H.J.; Jang, D.J. Development and evaluation of a reconstitutable dry suspension to improve the dissolution and oral absorption of poorly water-soluble celecoxib. Pharmaceutics 2018, 10, 140. [Google Scholar] [CrossRef] [PubMed]
- Song, W.H.; Yeom, D.W.; Lee, D.H.; Lee, K.M.; Yoo, H.J.; Chae, B.R.; Song, S.H.; Choi, Y.W. In situ intestinal permeability and in vivo oral bioavailability of celecoxib in supersaturating self-emulsifying drug delivery system. Arch. Pharmacal Res. 2014, 37, 626–635. [Google Scholar] [CrossRef]
- Eren, Z.S.; Tunçer, S.; Gezer, G.; Yildirim, L.T.; Banerjee, S.; Yilmaz, A. Improved solubility of celecoxib by inclusion in SBA-15 mesoporous silica: Drug loading in different solvents and release. Microporous Mesoporous Mater. 2016, 235, 211–223. [Google Scholar] [CrossRef]
- Kim, H.I.; Jee, J.P.; Kim, S.T.; Kang, D.; Kim, Y.C.; Kim, H.C.; Park, S.Y.; Lee, H.M.; Cho, K.H.; Kim, D.Y.; et al. Preparation and characterization of celecoxib nanosuspension using bead milling. J. Nanosci. Nanotechnol. 2019, 19, 1184–1187. [Google Scholar] [CrossRef]
- Cho, K.H.; Jee, J.P.; Yang, D.A.; Kim, S.T.; Kang, D.; Kim, D.Y.; Sim, T.; Park, S.Y.; Kim, K.; Jang, D.J. Improved dissolution and oral bioavailability of celecoxib by a dry elixir system. J. Nanosci. Nanotechnol. 2018, 18, 1482–1486. [Google Scholar] [CrossRef] [PubMed]
- Ha, E.S.; Choo, G.H.; Baek, I.H.; Kim, M.S. Formulation, characterization, and in vivo evaluation of celecoxib-PVP solid dispersion nanoparticles using supercritical antisolvent process. Molecules 2014, 19, 20325–20339. [Google Scholar] [CrossRef] [PubMed]
- Ha, E.S.; Ok, J.; Noh, J.; Jeong, H.Y.; Choo, G.H.; Jung, Y.S.; Baek, I.Y.; Kim, J.S.; Cho, W.; Hwang, S.J.; et al. Fabrication and evaluation of celecoxib microparticle surface modified by hydrophilic cellulose and surfactant. Int. J. Biol. Macromol. 2015, 72, 1473–1478. [Google Scholar] [CrossRef]
- Saffoon, N.; Uddin, R.; Huda, N.H.; Sutradhar, K.B. Enhancement of oral bioavailability and solid dispersion: A review. J. Appl. Pharm. Sci. 2011, 1, 13–20. [Google Scholar]
- Baghel, S.; Cathcart, H.; O’Reilly, N.J. Polymeric amorphous solid dispersions: A review of amorphization, crystallization, stabilization, solid-state characterization, and aqueous solubilization of biopharmaceutical classification system class II drugs. J. Pharm. Sci. 2016, 105, 2527–2544. [Google Scholar] [CrossRef] [PubMed]
- Jo, K.; Cho, J.M.; Lee, H.; Kim, E.K.; Kim, H.C.; Kim, H.; Lee, J. Enhancement of aqueous solubility and dissolution of celecoxib through phosphatidylcholine-based dispersion systems solidified with adsorbent carriers. Pharmaceutics 2019, 11, 1. [Google Scholar] [CrossRef]
- Jacobsen, A.C.; Elvang, P.A.; Bauer-Brandl, A.; Brandl, M. A dynamic in vitro permeation study on solid mono-and diacyl-phospholipid dispersions of celecoxib. Eur. J. Pharm. Sci. 2019, 127, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.S.; Balla, A.; Chun, K.H.; Chung, S.J.; Maeng, H.J. Physiologically-based pharmacokinetic modeling for drug-drug interactions of procainamide and N-acetylprocainamide with cimetidine, an inhibitor of rOCT2 and rMATE1, in rats. Pharmaceutics 2019, 11, 108. [Google Scholar] [CrossRef] [PubMed]
- Horvát, S.; Fehér, A.; Wolburg, H.; Sipos, P.; Veszelka, S.; Tóth, A.; Kis, L.; Kurunczi, A.; Balogh, G.; Kürti, L.; et al. Sodium hyaluronate as a mucoadhesive component in nasal formulation enhances delivery of molecules to brain tissue. Eur. J. Pharm. Biopharm. 2009, 72, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.J.; Kim, S.T.; Lee, K.; Oh, E. Improved bioavailability and antiasthmatic efficacy of poorly soluble curcumin-solid dispersion granules obtained using fluid bed granulation. Bio-Med. Mater. Eng. 2014, 24, 413–429. [Google Scholar]
- Hanke, U.; May, K.; Rozehnal, V.; Nagel, S.; Siegmund, W.; Weitschies, W. Commonly used nonionic surfactants interact differently with the human efflux transporters ABCB1 (p-glycoprotein) and ABCC2 (MRP2). Eur. J. Pharm. Biopharm. 2010, 76, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Mishra, G. Fluid Bed Technology: Overview and parameters for process selection. Int. J. Pharm. Sci. Drug Res. 2010, 2, 236–246. [Google Scholar]
- Sinha, V.R.; Anitha, R.; Ghosh, S.; Nanda, A.; Kumria, R. Complexation of celecoxib with β-cyclodextrin: Characterization of the interaction in solution and in solid state. J. Pharm. Sci. 2005, 94, 676–687. [Google Scholar] [CrossRef]
- Emery, E.; Oliver, J.; Pugsley, T.; Sharma, J.; Zhou, J. Flowability of moist pharmaceutical powders. Powder Technol. 2009, 189, 409–415. [Google Scholar] [CrossRef]
- Quispe-Condori, S.; Saldaña, M.D.A.; Temelli, F. Microencapsulation of flax oil with zein using spray and freeze drying. LWT-Food Sci. Technol. 2011, 44, 1880–1887. [Google Scholar] [CrossRef]
- Herting, M.G.; Kleinebudde, P. Roll compaction/dry granulation: Effect of raw material particle size on granule and tablet properties. Int. J. Pharm. 2007, 338, 110–118. [Google Scholar] [CrossRef]
- Shah, R.B.; Tawakkul, M.A.; Khan, M.A. Comparative evaluation of flow for pharmaceutical powders and granules. Aaps PharmSciTech 2008, 9, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Katstra, W.E.; Palazzolo, R.D.; Rowe, C.W.; Giritlioglu, B.; Teung, P.; Cima, M.J. Oral dosage forms fabricated by Three Dimensional Printing™. J. Control. Release 2000, 66, 1–9. [Google Scholar] [CrossRef]
- Iranloye, T.A.; Parrott, E.L. Effects of compression force, particle size, and lubricants on dissolution rate. J. Pharm. Sci. 1978, 67, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y. Effect of excipients on tablet properties and dissolution behavior of theophylline-tableted microcapsules under different compression forces. J. Pharm. Sci. 1988, 77, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, M.J.; Yoon, H.; Shim, C.R.; Ko, H.A.; Cho, S.A.; Lee, D.; Khang, G. Enhanced dissolution rate of celecoxib using PVP and/or HPMC-based solid dispersions prepared by spray drying method. J. Pharm. Investig. 2013, 43, 205–213. [Google Scholar] [CrossRef]
- Humberstone, A.J.; Charman, W.N. Lipid-based vehicles for the oral delivery of poorly water soluble drugs. Adv. Drug Deliv. Rev. 1997, 25, 103–128. [Google Scholar] [CrossRef]





| Solubilizer | Solubility (μg/mL) | Relative Ratio (Versus CXB, -Fold) |
|---|---|---|
| No additive (only CXB) | 2.0 ± 0.1 | - |
| Cremophor RH40 | 1434.7 ± 16.4 | 717.4 |
| Labrafac PG | 8.0 ± 2.7 | 4.0 |
| Labrafil M 1944 CS | 284.3 ± 21.3 | 142.2 |
| Labrasol | 1024.1 ± 27.9 | 512.1 |
| Plurol Oleique CC497 | 93.6 ± 12.2 | 46.8 |
| Poloxamer 188 | 25.0 ± 13.3 | 12.5 |
| Poloxamer 407 | 622.4 ± 22.0 | 311.2 |
| Ryoto sugar ester L-1695 | 329.3 ± 7.2 | 164.7 |
| Ryoto sugar ester P-1670 | 407.5 ± 0.6 | 203.8 |
| Soluplus | 823.1 ± 30.7 | 411.6 |
| SPAN 80 | 290.6 ± 157.8 | 145.3 |
| Transcutol HP | 44.8 ± 27.1 | 22.4 |
| Granules | Hausner Ratio | Carr’s Index (%) |
|---|---|---|
| Control (CXB) | 1.86 ± 0.10 | 46.0 ± 2.83 |
| SDG (CXB/Cre-RH = 1:0.1) | 1.14 ± 0.01 | 12.8 ± 0.6 |
| SDG (CXB/Cre-RH = 1:0.25) | 1.17 ± 0.01 | 14.7 ± 0.5 |
| SDG (CXB/Cre-RH = 1:0.5) | 1.23 ± 0.01 | 19.2 ± 0.6 |
| Tablets | Hardness (Kg/cm2) | Friability (%) |
| SDT (CXB/Cre-RH = 1:0.1) | 5.8 ± 0.6 | 0.51 |
| SDT (CXB/Cre-RH = 1:0.25) | 5.3 ± 0.3 | 0.45 |
| SDT (CXB/Cre-RH = 1:0.5) | 5.6 ± 0.6 | 0.50 |
| Pharmacokinetic Parameters | CXB Powder | Marketed Product (Celebrex®) | SDG (CXB/Cre-RH = 1:0.5) |
|---|---|---|---|
| Tmax (min) | 450 ± 60 | 220 ± 35 * | 450 ± 60 |
| Cmax (μg /mL) | 0.33 ± 0.03 | 1.56 ± 0.31 * | 1.62 ± 0.07 * |
| T1/2 (min) | 520 ± 203 | 317 ± 60 | 523 ± 146 |
| AUClast (μg∙min/mL) | 407 ± 54 | 1851 ± 386 * | 1888 ± 289 * |
| AUCinf (μg∙min/mL) | 423 ± 69 | 1853 ± 389 * | 1954 ± 353 * |
| MRT (min) | 949 ± 215 | 720 ± 39 | 840 ± 232 |
| Relative BA (%) | - | 438 | 462 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, H.J.; Heo, E.-J.; Kim, Y.-H.; Kim, S.; Hwang, Y.-H.; Byun, J.-M.; Cheon, S.H.; Park, S.Y.; Kim, D.Y.; Cho, K.H.; et al. Development and Evaluation of Poorly Water-Soluble Celecoxib as Solid Dispersions Containing Nonionic Surfactants Using Fluidized-Bed Granulation. Pharmaceutics 2019, 11, 136. https://doi.org/10.3390/pharmaceutics11030136
Kwon HJ, Heo E-J, Kim Y-H, Kim S, Hwang Y-H, Byun J-M, Cheon SH, Park SY, Kim DY, Cho KH, et al. Development and Evaluation of Poorly Water-Soluble Celecoxib as Solid Dispersions Containing Nonionic Surfactants Using Fluidized-Bed Granulation. Pharmaceutics. 2019; 11(3):136. https://doi.org/10.3390/pharmaceutics11030136
Chicago/Turabian StyleKwon, Hyeok Jin, Eun-Ji Heo, Young-Hwan Kim, Sarah Kim, Young-Ha Hwang, Ji-Mi Byun, Se Hyeop Cheon, Sang Yeob Park, Dong Yun Kim, Kwan Hyung Cho, and et al. 2019. "Development and Evaluation of Poorly Water-Soluble Celecoxib as Solid Dispersions Containing Nonionic Surfactants Using Fluidized-Bed Granulation" Pharmaceutics 11, no. 3: 136. https://doi.org/10.3390/pharmaceutics11030136