The Self-Assembly Phenomenon of Poloxamers and Its Effect on the Dissolution of a Poorly Soluble Drug from Solid Dispersions Obtained by Solvent Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods of Preparation of Solid Dispersions
2.2.1. Solvent Evaporation (E)
2.2.2. Spray Drying (SD)
2.2.3. Scanning Electron Microscopy (SEM)
2.2.4. Differential Scanning Calorimetry (DSC)
2.2.5. Powder X-ray Diffraction (PXRD)
2.2.6. Laser Diffraction Measurements
2.2.7. Fourier Transform Infrared Spectroscopy (FTIR)
2.2.8. Dynamic Light Scattering Measurements (DLS)
2.2.9. Emission Spectroscopy
2.2.10. Contact Angle Determination
2.2.11. Dissolution Study
3. Results and Discussion
3.1. Solid State Characterization
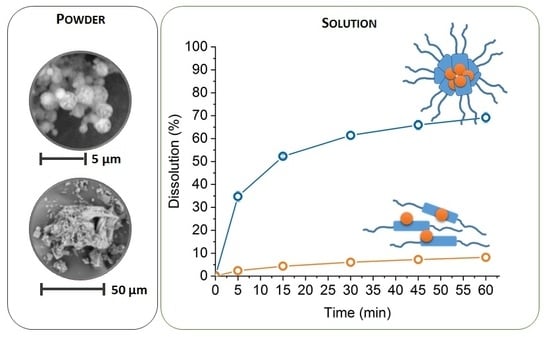
3.1.1. Size Distribution and Morphology of Particles of Solid Dispersions
3.1.2. X-Ray Powder Diffractometry (XRPD)
3.1.3. Vibrational Spectroscopy
3.1.4. Thermal Properties of Solid Dispersions
3.1.5. Wettability of Solid Dispersions
3.2. Characterization of Solid Dispersions in Solution
3.2.1. Self-Assembly of Poloxamers in Solid Dispersions
3.2.2. Solubilization of Molecular Probes
3.2.3. Dissolution Study
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Williams, H.D.; Trevaskis, N.L.; Charman, S.A.; Shanker, R.M.; Charman, W.N.; Pouton, C.W.; Porter, C.J.H. Strategies to Address Low Drug Solubility in Discovery and Development. Pharmacol. Rev. 2013, 65, 315–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di, L.; Kerns, E.H.; Carter, G.T. Drug-Like Property Concepts in Pharmaceutical Design. Curr. Pharm. Des. 2009, 15, 2184–2194. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, Y.; Wada, K.; Nakatani, M.; Yamada, S.; Onoue, S. Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: Basic approaches and practical applications. Int. J. Pharm. 2011, 420, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Krupa, A.; Descamps, M.; Willart, J.F.; Jachowicz, R.; Danède, F. High energy ball milling and supercritical carbon dioxide impregnation as co-processing methods to improve dissolution of tadalafil. Eur. J. Pharm. Sci. 2016, 95, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Rios-Doria, J.; Carie, A.; Costich, T.; Burke, B.; Skaff, H.; Panicucci, R.; Sill, K. A Versatile Polymer Micelle Drug Delivery System for Encapsulation and In Vivo Stabilization of Hydrophobic Anticancer Drugs. J. Drug Deliv. 2012, 951741. [Google Scholar] [CrossRef]
- Bader, R.A.; Silvers, A.L.; Zhang, N. Polysialic Acid-Based Micelles for Encapsulation of Hydrophobic Drugs. Biomacromolecules 2011, 12, 314–320. [Google Scholar] [CrossRef]
- Luo, R.; Venkatraman, S.S.; Neu, B. Layer-by-Layer Polyelectrolyte–Polyester Hybrid Microcapsules for Encapsulation and Delivery of Hydrophobic Drugs. Biomacromolecules 2013, 14, 2262–2271. [Google Scholar] [CrossRef]
- Szafraniec, J.; Błażejczyk, A.; Kuś, E.; Janik, M.; Zając, G.; Wietrzyk, J.; Chłopicki, S.; Zapotoczny, S. Robust oil-core nanocapsules with hyaluronate-based shells as promising nanovehicles for lipophilic compounds. Nanoscale 2017, 47. [Google Scholar] [CrossRef]
- Han, Y.; Shchukin, D.; Yang, J.; Simon, C.R.; Fuchs, H.; Möhwald, H. Biocompatible Protein Nanocontainers for Controlled Drugs Release. ACS Nano 2010, 4, 2838–2844. [Google Scholar] [CrossRef]
- Juère, E.; Florek, J.; Bouchoucha, M.; Jambhrunkar, S.; Wong, K.Y.; Popat, A.; Kleitz, F. In Vitro Dissolution, Cellular Membrane Permeability, and Anti-Inflammatory Response of Resveratrol-Encapsulated Mesoporous Silica Nanoparticles. Mol. Pharm. 2017, 14, 4431–4441. [Google Scholar] [CrossRef]
- Meka, A.K.; Jenkins, L.J.; Dàvalos-Salas, M.; Pujara, N.; Wong, K.Y.; Kumeria, T.; Mariadason, J.M.; Popat, A. Enhanced Solubility, Permeability and Anticancer Activity of Vorinostat Using Tailored Mesoporous Silica Nanoparticles. Pharmaceutics 2018, 10, 283. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, S.; Dewangan, A.K.; Pavurala, N. Enhanced dissolution of poorly soluble antiviral drugs from nanoparticles of cellulose acetate based solid dispersion matrices. Asian J. Pharm. Sci. 2017, 12, 532–541. [Google Scholar] [CrossRef]
- Kim, M.-S.; Baek, I. Fabrication and evaluation of valsartan-polymer-surfactant composite nanoparticles by using the supercritical antisolvent process. Int. J. Nanomed. 2014, 9, 5167–5176. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.H.; Poudel, B.K.; Marasini, N.; Chi, S.-C.; Choi, H.-G.; Yong, C.S.; Kim, J.O. Preparation and evaluation of raloxifene-loaded solid dispersion nanoparticle by spray-drying technique without an organic solvent. Int. J. Pharm. 2013, 443, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Van den Moter, G. The use of amorphous solid dispersions: A formulation strategy to overcome poor solubility and dissolution rate. Drug Discov. Today 2012, 9, e79–e85. [Google Scholar] [CrossRef] [PubMed]
- Le-Ngoc Vo, C.; Park, C.; Lee, B.-J. Current trends and future perspectives of solid dispersions containing poorly water-soluble drugs. Eur. J. Pharm. Biopharm. 2013, 85, 799–813. [Google Scholar] [CrossRef]
- Maniruzzaman, M.; Pang, J.; Morgan, D.J.; Douroumis, D. Molecular modeling as a predictive tool for the development of solid dispersions. Mol. Pharm. 2015, 12, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, T.; Marques, S.; das Neves, J.; Sarmento, B. Amorphous solid dispersions: Rational selection of a manufacturing process. Adv. Drug Deliv. Rev. 2016, 100, 85–101. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, K.; Obi, N. Studies on absorption of eutectic mixture. I. A comparison of the behavior of eutectic mixture of sulfathiazole and that of ordinary sulfathiazole in man. Chem. Pharm. Bull. 1961, 9, 866–872. [Google Scholar] [CrossRef]
- Sekiguchi, K.; Obi, N.; Ueda, Y. Studies on absorption of eutectic mixture. II. Absorption of fused conglomerates of chloramphenicol and urea in rabbits. Chem. Pharm. Bull. 1961, 12, 134–144. [Google Scholar] [CrossRef]
- Goldberg, A.H.; Gibaldi, M.; Kanig, J.L. Increasing dissolution rates and gastrointestinal absorption of drugs via solid solutions and eutectic mixtures. I. Theoretical considerations and discussion of the literature. J. Pharm. Sci. 1965, 54, 1145–1148. [Google Scholar] [CrossRef] [PubMed]
- Chiou, W.L.; Riegelman, S. Pharmaceutical applications of solid dispersion systems. J. Pharm. Sci. 1971, 60, 1281–1302. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Jain, C.P. Preparation and characterization of solid dispersions of carvedilol with PVP K30. Res. Pharm. Sci. 2010, 5, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Kakumanu, V.K.; Bansal, A.K. Stability and solubility of celecoxib-PVP amorphous dispersions: A molecular perspective. Pharm. Res. 2004, 21, 1762–1769. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.-T.; Balakrishnan, P.; Oh, D.H.; Joe, K.H.; Kim, Y.R.; Hwang, D.H.; Lee, Y.-B.; Yong, C.S.; Choi, H.-G. Development of novel sibutramine base-loaded solid dispersion with gelatin and HPMC: Physicochemical characterization and pharmacokinetics in beagle dogs. Int. J. Pharm. 2010, 397, 225–230. [Google Scholar] [CrossRef]
- Alonzo, D.E.; Gao, Y.; Zhou, D.; Mo, H.; Zhang, G.G.Z.; Taylor, L.S. Dissolution and precipitation behavior of amorphous solid dispersions. J. Pharm. Sci. 2011, 100, 3316–3331. [Google Scholar] [CrossRef] [PubMed]
- Frank, K.J.; Rosenblatt, K.M.; Westedt, U.; Holig, P.; Rosenberg, J.; Magerlein, M.; Fricker, G.; Brandl, M. Amorphous solid dispersion enhances permeation of poorly soluble ABT-102: True supersaturation vs. apparent solubility enhancement. Int. J. Pharm. 2012, 437, 288–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, D.D.; Lee, P.I. Evolution of supersaturation of amorphous pharmaceuticals: The effect of rate of supersaturation generation. Mol. Pharm. 2013, 10, 4330–4346. [Google Scholar] [CrossRef]
- Chauhan, H.; Hui-Gu, C.; Atef, E. Correlating the behavior of polymers in solution as precipitation inhibitor to its amorphous stabilization ability in solid dispersions. J. Pharm. Sci. 2013, 102, 1924–1935. [Google Scholar] [CrossRef]
- Passerini, N.; Albertini, B.; González-Rodríguez, M.L.; Cavallari, C.; Rodriguez, L. Preparation and characterisation of ibuprofen–poloxamer 188 granules obtained by melt granulation. Eur. J. Pharm. Sci. 2002, 15, 71–78. [Google Scholar] [CrossRef]
- Eloy, J.O.; Marchetti, J.M. Solid dispersions containing ursolic acid in Poloxamer 407 and PEG 6000: A comparative study of fusion and solvent methods. Powder Technol. 2014, 253, 98–106. [Google Scholar] [CrossRef]
- Joshi, H.N.; Tejwani, R.W.; Davidovich, M.; Sahasrabudhe, V.P.; Jemal, M.; Bathala, M.S.; Varia, S.A.; Serajuddin, A. Bioavailability enhancement of a poorly water-soluble drug by solid dispersion in polyethylene glycol–polysorbate 80 mixture. Int. J. Pharm. 2004, 269, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Moes, J.; Koolen, S.; Huitema, A.; Schellens, J.; Beijnen, J.; Nuijen, B. Pharmaceutical development and preliminary clinical testing of an oral solid dispersion formulation of docetaxel (ModraDoc001). Int. J. Pharm. 2011, 420, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Ghebremeskel, A.N.; Vemavarapu, C.; Lodaya, M. Use of surfactants as plasticizers in preparing solid dispersions of poorly soluble API: Stability testing of selected solid dispersions. Pharm. Res. 2006, 23, 1928–1936. [Google Scholar] [CrossRef] [PubMed]
- Chaves, L.L.; Vieira, A.C.C.; Reis, S.; Sarmento, B.; Ferreira, D.C. Quality by Design: Discussing and Assessing the Solid Dispersions Risk. Curr. Drug Deliv. 2014, 11, 253–269. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Jain, A.K.; Singh, C.; Kumar, R. Development, characterization and solubility study of solid dispersion of terbinafine hydrochloride by solvent evaporation method. Asian J. Pharm. 2008, 2, 154–158. [Google Scholar] [CrossRef]
- Tabbakhian, M.; Hasanzadeh, F.; Tavakoli, N.; Jamshidian, Z. Dissolution enhancement of glibenclamide by solid dispersion: Solvent evaporation versus a supercritical fluid-based solvent-antisolvent technique. Res. Pharm. Sci. 2014, 9, 337–350. [Google Scholar] [PubMed]
- Przybyłek, M.; Ziółkowska, D.; Mroczyńska, K.; Cysewski, P. Propensity of salicylamide and ethenzamide cocrystallization with aromatic carboxylic acids. Eur. J. Pharm. Sci. 2016, 31, 132–140. [Google Scholar] [CrossRef]
- Przybyłek, M.; Cysewski, P.; Pawelec, M.; Ziółkowska, D.; Kobierski, M. On the origin of surface imposed anisotropic growth of salicylic and acetylsalicylic acids crystals during droplet evaporation. J. Mol. Model. 2015, 21, 49. [Google Scholar] [CrossRef]
- Al-Ali, M.; Periasamy, S.; Parthasarathy, R. Novel drying of formulated naproxen sodium using microwave radiation: Characterization and energy comparison. Powder Technol. 2018, 334, 143–150. [Google Scholar] [CrossRef]
- Lee, H.; Lee, J. Dissolution enhancement of celecoxib via polymer-induced crystallization. J. Cryst. Growth 2013, 374, 37–42. [Google Scholar] [CrossRef]
- Bajaj, A.; Rao, M.R.P.; Pardeshi, A.; Sali, D. Nanocrystallization by Evaporative Antisolvent Technique for Solubility and Bioavailability Enhancement of Telmisartan. AAPS Pharm. Sci. Technol. 2012, 13, 1331–1340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, Z.; Zha, X.; Wang, Z.; Wang, X.; Fan, W. Response Surface Modeling of Drug-Loaded Micelles Prepared Through Supercritical Carbon Dioxide Evaporation Method Using Box-Behnken Experimental Design. J. Nanosci. Nanotechnol. 2019, 19, 3616–3620. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Ali, M.; Baboota, S.; Ahuja, A.; Kumar, A.; Ali, J. Solid Dispersion as an Approach for Bioavailability Enhancement of Poorly Water-Soluble Drug Ritonavir. AAPS Pharm. Sci. Technol. 2010, 11, 518–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vehring, R. Pharmaceutical particle engineering via spray drying. Pharm. Res. 2008, 25, 999–1022. [Google Scholar] [CrossRef]
- Ansari, M.T.; Karim, S.; Ranjha, N.M.; Shah, N.H.; Muhammad, S. Physicochemical characterization of artemether solid dispersions with hydrophilic carriers by freeze dried and melt methods. Arch. Pharm. Res. 2010, 33, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Van den Mooter, G. Spray drying formulation of amorphous solid dispersions. Adv. Drug Del. Rev. 2016, 100, 27–50. [Google Scholar] [CrossRef] [PubMed]
- Paudel, A.; Worku, A.; Meeus, J.; Guns, S.; Mooter, G. Manufacturing of solid dispersions of poorly water soluble drugs by spray drying: Formulation and process considerations. Int. J. Pharm. 2013, 453, 253–284. [Google Scholar] [CrossRef]
- Kolašinac, N.; Kachrimanis, K.; Homšek, I.; Grujić, B.; Ðurić, Z.; Ibrić, S. Solubility enhancement of desloratadine by solid dispersion in poloxamers. Int. J. Pharm. 2012, 436, 161–170. [Google Scholar] [CrossRef]
- Shah, T.J.; Amin, A.F.; Parikh, J.R.; Parikh, R.H. Process Optimization and Characterization of Poloxamer Solid Dispersions of a Poorly Water-soluble Drug. AAPS Pharm. Sci. Technol. 2007, 8, E18–E24. [Google Scholar] [CrossRef]
- Masiello, D.; Cheng, S.; Bubley, G.J.; Lu, M.L.; Balk, S.P. Bicalutamide Functions as an Androgen Receptor Antagonist by Assembly of a Transcriptionally Inactive Receptor. J. Biol. Chem. 2002, 227, 26321–26326. [Google Scholar] [CrossRef] [PubMed]
- Kumbhar, D.D.; Pokharkar, V.B. Engineering of nanostructured lipid carrier for the poorly water-soluble drug, bicalutamide: Physicochemical investigations. Colloids Surf. A 2013, 416, 32–42. [Google Scholar] [CrossRef]
- Le, Y.; Chen, J.-F.; Shen, Z.; Yun, J.; Pu, M. Nanosized bicalutamide and its molecular structure in solvents. Int. J. Pharm. 2009, 370, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Vega, D.R.; Polla, G.; Martinez, A.; Mendioroz, E.; Reinoso, M. Conformational polymorphism in bicalutamide. Int. J. Pharm. 2007, 328, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.R.; Gu, J.M. N-[4-Cyano-3-trifluoromethyl)phenyl]-3-(4-fluorophenylsulfonyl)-2-hydroxy-2-methylpropionamide. Acta Cryst. E61 2005, 3897–3898. [Google Scholar] [CrossRef]
- Perlovich, G.L.; Blokhina, S.V.; Manin, N.G.; Volkova, T.V.; Tkachev, V.V. Polymorphism and solvatomorphism of bicalutamide. Thermophysical study and solubility. J. Therm. Anal. Calorim. 2013, 11, 655–662. [Google Scholar] [CrossRef]
- Szafraniec, J.; Antosik, A.; Knapik-Kowalczuk, J.; Kurek, M.; Syrek, K.; Chmiel, K.; Paluch, M.; Jachowicz, R. Planetary ball milling and supercritical fluid technology as a way to enhance dissolution of bicalutamide. Int. J. Pharm. 2017, 533, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Szafraniec, J.; Antosik, A.; Knapik-Kowalczuk, J.; Chmiel, K.; Kurek, M.; Gawlak, K.; Paluch, M.; Jachowicz, R. Enhanced dissolution of solid dispersions containing bicalutamide subjected to mechanical stress. Int. J. Pharm. 2018, 542, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, M.V.; Murali Mohan Babu, G.V.; Sunil, S.A.; Sreenivasa Rao, N.; Ramana Murthy, K.V. In-vitro dissolution rate enhancement of poorly water soluble non-steroidal antiandrogen agent, bicalutamide, with hydrophilic carrier. J. Sci. Ind. Res. 2010, 69, 629–634. [Google Scholar]
- Szafraniec, J.; Antosik, A.; Knapik-Kowalczuk, J.; Gawlak, K.; Kurek, M.; Szlęk, J.; Jamróz, W.; Paluch, M.; Jachowicz, R. Molecular Disorder of Bicalutamide—Amorphous Solid Dispersions Obtained by Solvent Methods. Pharmaceutics 2018, 10, 194. [Google Scholar] [CrossRef]
- Szczurek, J.; Rams-Baron, M.; Knapik-Kowalczuk, J.; Antosik, A.; Szafraniec, J.; Jamróz, W.; Dulski, M.; Jachowicz, R.; Paluch, M. Molecular Dynamics, Recrystallization Behavior, and Water Solubility of the Amorphous Anticancer Agent Bicalutamide and Its Polyvinylpyrrolidone Mixtures. Mol. Pharm. 2017, 14, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Puri, K.; Dantuluri, A.K.; Kumar, M.; Karar, N.; Bansal, A.K. Wettability and surface chemistry of crystalline and amorphous forms of a poorly water soluble drug. Eur. J. Pharm. Sci. 2010, 40, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Bérard, V.; Lesniewska, E.; Andrès, C.; Pertuy, D.; Laroche, C.; Pourcelot, Y. Affinity scale between a carrier and a drug in DPI studied by atomic force microscopy. Int. J. Pharm. 2002, 247, 127–137. [Google Scholar] [CrossRef]
- Yusa, S.; Sakakibara, A.; Yamamoto, T.; Morishima, M. Reversible pH-Induced Formation and Disruption of Unimolecular Micelles of an Amphiphilic Polyelectrolyte. Macromolecules 2002, 35, 5243–5249. [Google Scholar] [CrossRef]
- Rymarczyk-Machal, M.; Szafraniec, J.; Zapotoczny, S.; Nowakowska, M. Photoactive graft amphiphilic polyelectrolyte: Facile synthesis, intramolecular aggregation and photosensitizing activity. Eur. Polym. J. 2014, 55, 76–85. [Google Scholar] [CrossRef]
- McKenzie, B.E.; de Visser, J.F.; Friedrich, H.; Wirix, M.J.M.; Bomans, P.H.H.; de With, G.; Holder, S.J.; Sommerdijk, N.A.J.M. Bicontinuous Nanospheres from Simple Amorphous Amphiphilic Diblock Copolymers. Macromolecules 2013, 46, 9845–9848. [Google Scholar] [CrossRef]
- Dumortier, G.; Grossiord, J.L.; Agnely, F.; Chaumeil, J.C. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm. Res. 2006, 23, 2709–2728. [Google Scholar] [CrossRef]
- Rowe, R.C.; Sheskey, P.J.; Quinn, M.E. (Eds.) Handbook of Pharmaceutical Excipients, 6th ed.; Pharmaceuicall Press: London, UK, 2009; pp. 506–509. [Google Scholar]
- Miller, M.M.; Wasik, S.P.; Huang, G.L.; Siu, W.Y.; Mackay, D. Relationships between octanol-water partition coefficient and aqueous solubility. Environ. Sci. Technol. 1985, 19, 522–529. [Google Scholar] [CrossRef]
- Capek, I. Fate of excited probes in micellar systems. Adv. Colloid Interface Sci. 2002, 97, 91–149. [Google Scholar] [CrossRef]
- Barry, N.P.E.; Therrien, B. Organic Nanoreactors. Pyrene: The Guest of Honor. In From Molecular to Supramolecular Organic Compound; Sadjadi, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 421–461. [Google Scholar]
- Ren, F.; Jing, Q.; Tang, Y.; Shen, Y.; Chen, J.; Gao, F.; Cui, J. Characteristics of bicalutamide solid dispersions and improvement of the dissolution. Drug Dev. Ind. Pharm. 2006, 32, 967–972. [Google Scholar] [CrossRef]
- Sancheti, P.P.; Vyas, V.M.; Shah, M.; Karekar, P.; Pore, Y.V. Development and characterization of bicalutamide-poloxamer F68 solid dispersion systems. Pharmazie 2008, 63, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Antosik, A.; Witkowski, S.; Woyna-Orlewicz, K.; Talik, P.; Szafraniec, J.; Wawrzuta, B.; Jachowicz, R. Application of supercritical carbon dioxide to enhance dissolution rate of bicalutamide. Acta Pol. Pharm. 2017, 74, 1231–1238. [Google Scholar]













| Method | Carrier | BCL:polymers wt. Ratio | Dx(50) ± SD (µm) | Span |
|---|---|---|---|---|
| Evaporation | PLX188 | 1:1 | 247.0 ± 55.9 | 4.698 |
| 2:1 | 227.0 ± 30.7 | 2.719 | ||
| PLX407 | 1:1 | 203.0 ± 23.1 | 2.971 | |
| 2:1 | 159.0 ± 53.1 | 4.438 | ||
| Spray-drying | PLX188 | 1:1 | 196.0 ± 27.2 | 4.405 |
| 2:1 | 355.0 ± 64.7 | 2.876 | ||
| PLX407 | 1:1 | 445.0 ± 28.7 | 1.771 | |
| 2:1 | 154.0 ± 13.7 | 2.167 | ||
| PLX188-PVP | 2:1:1 | 78.0 ± 0.8 | 3.339 | |
| 4:1:1 | 55.6 ± 1.5 | 3.907 | ||
| PLX407-PVP | 2:1:1 | 48.6 ± 2.2 | 5.153 | |
| 4:1:1 | 54.2 ± 2.1 | 4.098 |
| System | Tg-PLX (°C) (midpoint) | Tg-PVP (°C) (midpoint) | Tm1 (°C) (onset) | ΔHm1 (J/g) | Tm2 (°C) (onset) | ΔHm2 (J/g) |
|---|---|---|---|---|---|---|
| Raw BCL | - | - | - | - | 194 | 110.8 |
| Raw PVP | - | 172 | - | - | - | - |
| Raw PLX 188 | −60 | - | 53 | 134.9 | - | - |
| Raw PLX 407 | −65 | - | 56 | 117.3 | - | - |
| BCL-PLX 188 1:1 E | −60 | - | 50 | 65.7 | 160 | 39.6 |
| BCL-PLX 188 2:1 E | −61 | - | 49 | 45.7 | 176 | 42.4 |
| BCL-PLX 188 1:1 SD | −61 | - | 49 | 66.6 | 160 | 39.2 |
| BCL-PLX 188 2:1 SD | −61 | - | 48 | 42.3 | 176 | 42.6 |
| BCL-PLX 407 1:1 E | −65 | - | 52 | 59.6 | 160 | 39.5 |
| BCL-PLX 407 2:1 E | −65 | - | 52 | 39.2 | 182 | 52.4 |
| BCL-PLX 407 1:1 SD | −66 | - | 52 | 58.9 | 165 | 39.6 |
| BCL-PLX 407 2:1 SD | −66 | - | 52 | 38.9 | 181 | 49.2 |
| BCL-PLX-PVP 188 2:1:1 SD | −61 | 142 | 47 | 188 | 135 | 27 |
| BCL-PLX-PVP 188 4:1:1 SD | −62 | 146 | 45 | 120 | 134 | 50 |
| BCL-PLX-PVP 407 2:1:1 SD | −67 | 141 | 47 | 140 | 135 | 26 |
| BCL-PLX-PVP 407 4:1:1 SD | −67 | 142 | 47 | 95 | 137 | 48 |
| System | IIII/II | System | IIII/II |
|---|---|---|---|
| Water | 0.351 | BCL-PLX 188 1:1 (SD) | 0.442 |
| Raw PLX188 | 0.516 | BCL-PLX 188 2:1 (SD) | 0.430 |
| Raw PLX407 | 0.503 | BCL-PLX 407 1:1 (SD) | 0.435 |
| BCL-PLX 407 2:1 (SD) | 0.442 | ||
| BCL-PLX 188 1:1 (E) | 0.508 | BCL-PLX 188-PVP 2:1:1 (SD) | 0.556 |
| BCL-PLX 188 2:1 (E) | 0.523 | BCL-PLX 188-PVP 4:1:1 (SD) | 0.628 |
| BCL-PLX 407 1:1 (E) | 0.441 | BCL-PLX 407-PVP 2:1:1 (SD) | 0.661 |
| BCL-PLX 407 2:1 (E) | 0.512 | BCL-PLX 407-PVP 4:1:1 (SD) | 0.670 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szafraniec, J.; Antosik, A.; Knapik-Kowalczuk, J.; Chmiel, K.; Kurek, M.; Gawlak, K.; Odrobińska, J.; Paluch, M.; Jachowicz, R. The Self-Assembly Phenomenon of Poloxamers and Its Effect on the Dissolution of a Poorly Soluble Drug from Solid Dispersions Obtained by Solvent Methods. Pharmaceutics 2019, 11, 130. https://doi.org/10.3390/pharmaceutics11030130
Szafraniec J, Antosik A, Knapik-Kowalczuk J, Chmiel K, Kurek M, Gawlak K, Odrobińska J, Paluch M, Jachowicz R. The Self-Assembly Phenomenon of Poloxamers and Its Effect on the Dissolution of a Poorly Soluble Drug from Solid Dispersions Obtained by Solvent Methods. Pharmaceutics. 2019; 11(3):130. https://doi.org/10.3390/pharmaceutics11030130
Chicago/Turabian StyleSzafraniec, Joanna, Agata Antosik, Justyna Knapik-Kowalczuk, Krzysztof Chmiel, Mateusz Kurek, Karolina Gawlak, Joanna Odrobińska, Marian Paluch, and Renata Jachowicz. 2019. "The Self-Assembly Phenomenon of Poloxamers and Its Effect on the Dissolution of a Poorly Soluble Drug from Solid Dispersions Obtained by Solvent Methods" Pharmaceutics 11, no. 3: 130. https://doi.org/10.3390/pharmaceutics11030130