Design and Evaluation of An Extended-Release Olmesartan Tablet Using Chitosan/Cyclodextrin Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Measurements of Turbidity
2.3. Solubility Studies
2.4. Fourier Transform Infrared (FT-IR) Spectroscopy Study
2.5. Preparation of SD-CS/CDs/OLM Extended-Release Tablets
2.6. In Vitro Dissolution Studies of SD-CS/CDs/OLM Composite
2.7. In Vivo Pharmacokinetic Studies of SD-CS/CDs/OLM Composite
2.8. Antihypertensive Studies of Spray-dried Chitosan Cyclodextrins (SD-CS/CDs)/Olmesartan Composite
2.9. Statistics
3. Results and Discussion
3.1. Characterization of Spray-dried Chitosan Cyclodextrins (SD-CS/CD) Composites
3.1.1. Turbidity Measurements
3.1.2. FT-IR Measurements
3.1.3. Solubility Studies
3.2. In Vitro Release of OLM
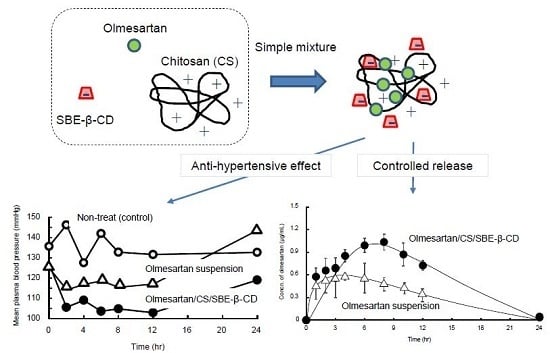
3.3. In Vivo Release of Olmesartan Tablets (OLM)
3.4. In Vivo Antihypertensive Effects of OLM
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chrysant, S.G. Effectiveness of the fixed-dose combination of olmesartan/amlodipine/hydrochlorothiazide for the treatment of hypertension in patients stratified by age, race and diabetes, CKD and chronic CVD. Expert. Rev. Cardiovasc Ther. 2013, 11, 1115–1124. [Google Scholar] [CrossRef] [PubMed]
- Brousil, J.A.; Burke, J.M. Olmesartan medoxomil: An angiotensin II-receptor blocker. Clin. Ther. 2003, 25, 1041–1055. [Google Scholar] [CrossRef]
- Kadowaki, D.; Anraku, M.; Tasaki, Y.; Kitamura, K.; Wakamatsu, S.; Tomita, K.; Gebicki, J.M.; Maruyama, T.; Otagiri, M. Effect of olmesartan on oxidative stress in hemodialysis patients. Hypertens Res. 2007, 30, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, D.; Anraku, M.; Tasaki, Y.; Taguchi, K.; Shimoishi, K.; Seo, H.; Hirata, S.; Maruyama, T.; Otagiri, M. Evaluation for antioxidant and renoprotective activity of olmesartan using nephrectomy rats. Biol. Pharm. Bull 2009, 32, 2041–2045. [Google Scholar] [CrossRef]
- Kadowaki, D.; Anraku, M.; Sakaya, M.; Hirata, S.; Maruyama, T.; Otagiri, M. Olmesartan protects endothelial cells against oxidative stress-mediated cellular injury. Clin Exp Nephrol. 2015, 19, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Fuentes, M.; Alonso, M.J. Chitosan-based drug nanocarriers: Where do we stand? J. Control Release 2012, 161, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Anraku, M.; Gebicki, J.M.; Iohara, D.; Tomida, H.; Uekama, K.; Maruyama, T.; Hirayama, F.; Otagiri, M. Antioxidant activities of chitosans and its derivatives in in vitro and in vivo studies. Carbohydr. Polym. 2018, 199, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Trapani, A.; Lopedota, A.; Franco, M.; Cioffi, N.; Ieva, E.; Garcia-Fuentes, M.; Alonso, M.J. A comparative study of chitosan and chitosan/cyclodextrin nanoparticles as potential carriers for the oral delivery of small peptides. Eur J. Pharm. Biopharm. 2010, 75, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Trapani, A.; Garcia-Fuentes, M.; Alonso, M.J. Novel drug nanocarriers combining hydrophilic cyclodextrins and chitosan. Nanotechnology 2008, 19, 185101. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, M.; Seijo, B.; Alonso, M.J. Novel hyaluronic acid-chitosan nanoparticles for ocular gene therapy. Invest. Ophthalmol. Vis. Sci. 2008, 49, 2016–2024. [Google Scholar] [CrossRef]
- Maestrelli, F.; Garcia-Fuentes, M.; Mura, P.; Alonso, M.J. A new drug nanocarrier consisting of chitosan and hydoxypropylcyclodextrin. Eur. J. Pharm. Biopharm. 2006, 63, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Savolainen, J.; Järvinen, K.; Matilainen, L.; Järvinen, T. Improved dissolution and bioavailability of phenytoin by sulfobutylether-β-cyclodextrin (SBE)7M-β-CD) and hydroxypropyl-β-cyclodextrin (HP-β-CD) complexation. Int. J. Pharm. 1998, 165, 69–78. [Google Scholar] [CrossRef]
- Mady, F.M.; Abou-Taleb, A.E.; Khaled, K.A.; Yamasaki, K.; Iohara, D.; Ishiguro, T.; Hirayama, F.; Uekama, K.; Otagiri, M. Enhancement of the aqueous solubility and masking the bitter taste of famotidine using drug/SBE-beta-CyD/povidone K30 complexation approach. J. Pharm. Sci. 2010, 99, 4285–4294. [Google Scholar] [CrossRef] [PubMed]
- Devasari, N.; Dora, C.P.; Singh, C.; Paidi, S.R.; Kumar, V.; Sobhia, M.E.; Suresh, S. Inclusion complex of erlotinib with sulfobutyl ether-β-cyclodextrin: Preparation, characterization, in silico, in vitro and in vivo evaluation. Carbohydr Polym. 2015, 134, 547–556. [Google Scholar] [CrossRef]
- Anraku, M.; Hiraga, A.; Iohara, D.; Pipkin, J.D.; Uekama, K.; Hirayama, F. Slow-release of famotidine from tablets consisting of chitosan/sulfobutyl ether β-cyclodextrin composites. Int. J. Pharm. 2015, 487, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Arima, H.; Kihara, F.; Hirayama, F.; Uekama, K. Enhancement of gene expression by polyamidoamine dendrimer conjugates with alpha-, beta-, and gamma-cyclodextrins. Bioconjug. Chem. 2001, 12, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T.; Connors, K.A. Phase-solubility techniques. Advan. Anal Chem. Instr. 1965, 117–212. [Google Scholar]
- Brewster, M.E.; Loftsson, T. Cyclodextrins as pharmaceutical solubilizers. Adv. Drug Deliv. Rev. 2007, 59, 645–666. [Google Scholar] [CrossRef]
- Anraku, M.; Hiraga, A.; Iohara, D.; Uekama, K.; Tomida, H.; Otagiri, M.; Hirayama, F. Preparation and antioxidant activity of PEGylated chitosans with different particle sizes. Int. J. Biol. Macromol. 2014, 70, 64–69. [Google Scholar] [CrossRef]
- Beg, S.; Sharma, G.; Thanki, K.S.; Jain, O.P.; Katare, B. Singh, Positively charged self-nanoemulsifying oily formulations of olmesartan medoxomil: Systematic development, in vitro, ex vivo and in vivo evaluation. Int. J. Pharm. 2015, 493, 466–482. [Google Scholar] [CrossRef]
- Yamaoka, K.; Tanigawara, Y.; Nakagawa, T.; Uno, T. A pharmacokinetic analysis program (multi) for microcomputer. J. Pharmacobiodyn. 1981, 4, 879–885. [Google Scholar] [CrossRef]
- de la Torre, P.M.; Enobakhare, Y.; Torrado, G.; Torrado, S. Release ofamoxicillin from polyionic complexes of chitosan and poly(acrylic acid) Study of polymer/polymer and polymer/drug interactions within the network structure. Biomaterials 2003, 24, 1499–1506. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, X.; Hu, W.; Bai, Y.; Zhang, L. Preparation and evaluation of naringenin-loaded sulfobutylether-β-cyclodextrin/chitosan nanoparticles for ocular drug delivery. Carbohydr Polym. 2016, 149, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Uekama, K.; Hirayama, F.; Irie, T. Cyclodextrin Drug Carrier Systems. Chem. Rev. 1998, 98, 2045–2076. [Google Scholar] [CrossRef] [PubMed]
- Uekama, K.; Hieda, Y.; Hirayama, F.; Arima, H.; Sudoh, M.; Yagi, A.; Terashima, H. Stabilizing and solubilizing effects of sulfobutyl ether beta-cyclodextrin on prostaglandin E1 analogue. Pharm. Res. 2001, 18, 1578–1585. [Google Scholar] [CrossRef] [PubMed]
- García-Río, L.; Méndez, M.; Paleo, M.R.; Sardina, F.J. New insights in cyclodextrin: surfactant mixed systems from the use of neutral and anionic cyclodextrin derivatives. J. Phys. Chem. B 2007, 111, 12756–12764. [Google Scholar] [CrossRef] [PubMed]
- Lockwood, S.F.; O’Malley, S.; Mosher, G.L. Improved aqueous solubility of crystalline astaxanthin (3,3′-dihydroxy-β, β-carotene-4,4′-dione) by captisol® (sulfobutyl ether β-cyclodextrin). J. Pharm. Sci. 2003, 92, 922–926. [Google Scholar] [CrossRef]
- Anraku, M.; Iohara, D.; Hiraga, A.; Uekama, K.; Ifuku, S.; Pipkin, J.D.; Hirayama, F. Formation of elastic gels from deacetylated chitin nanofibers reinforced with sulfobutyl ether β-cyclodextrin. Chem. Lett. 2015, 44, 285–287. [Google Scholar] [CrossRef]
- Tabuchi, R.; Anraku, M.; Iohara, D.; Ishiguro, T.; Ifuku, S.; Nagae, T.; Uekama, K.; Okazaki, S.; Takeshita, K.; Otagiri, M.; et al. Surface-deacetylated chitin nanofibers reinforced with a sulfobutyl ether β-cyclodextrin gel loaded with prednisolone as potential therapy for inflammatory bowel disease. Carbohydr Polym. 2017, 174, 1087–1094. [Google Scholar] [CrossRef]






| AUC(0-∞) (mgh/mL) | Cmax (μg/mL) | T1/2 (h) | Tmax (h) | |
|---|---|---|---|---|
| OLM suspension | 7.8 ± 0.10 | 0.80 ± 0.16 | 3.6 ± 0.11 | 3.3 ± 0.16 |
| OLM/SD-CS/lactose | 8.2 ± 0.80 | 0.74 ± 0.12 | 4.5 ± 0.34 | 3.6 ± 0.17 |
| OLM/SD-CS/HP-β-CD | 9.4 ± 0.58 b | 1.0 ± 0.033 a | 3.0 ± 0.45 | 3.3 ± 0.26 |
| OLM/SD-CS/SBE-β-CD | 14.1 ± 1.2 a,d,e | 1.0 ± 0.10 b,d | 4.2 ± 0.29 b,e | 5.0 ± 0.10 b,c,e |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anraku, M.; Tabuchi, R.; Goto, M.; Iohara, D.; Mizukai, Y.; Maezaki, Y.; Michihara, A.; Kadowaki, D.; Otagiri, M.; Hirayama, F. Design and Evaluation of An Extended-Release Olmesartan Tablet Using Chitosan/Cyclodextrin Composites. Pharmaceutics 2019, 11, 82. https://doi.org/10.3390/pharmaceutics11020082
Anraku M, Tabuchi R, Goto M, Iohara D, Mizukai Y, Maezaki Y, Michihara A, Kadowaki D, Otagiri M, Hirayama F. Design and Evaluation of An Extended-Release Olmesartan Tablet Using Chitosan/Cyclodextrin Composites. Pharmaceutics. 2019; 11(2):82. https://doi.org/10.3390/pharmaceutics11020082
Chicago/Turabian StyleAnraku, Makoto, Ryo Tabuchi, Miwa Goto, Daisuke Iohara, Yasuyuki Mizukai, Yuji Maezaki, Akihiro Michihara, Daisuke Kadowaki, Masaki Otagiri, and Fumitoshi Hirayama. 2019. "Design and Evaluation of An Extended-Release Olmesartan Tablet Using Chitosan/Cyclodextrin Composites" Pharmaceutics 11, no. 2: 82. https://doi.org/10.3390/pharmaceutics11020082