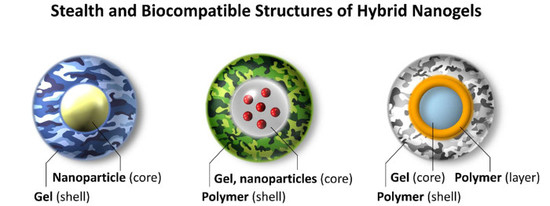
Hybrid Nanogels: Stealth and Biocompatible Structures for Drug Delivery Applications
Abstract
:1. Introduction
2. Polyethylene Glycol Decoration
3. Nanogels inside Liposomes: Nanolipogels
4. Zwitterionic Nanogels
5. Challenges and New Perspectives for Hybrid Nanogels
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Oh, J.K.; Drumright, R.; Siegwart, D.J.; Matyjaszewski, K. The development of microgels/nanogels for drug delivery applications. Prog. Polym. Sci. 2008, 33, 448–477. [Google Scholar] [CrossRef]
- Karimi, M.; Ghasemi, A.; Sahandi Zangabad, P.; Rahighi, R.; Moosavi Basri, S.M.; Mirshekari, H.; Amiri, M.; Shafaei Pishabad, Z.; Aslani, A.; Bozorgomid, M.; et al. Smart micro/nanoparticles in stimulus-responsive drug/gene delivery systems. Chem. Soc. Rev. 2016, 45, 1457–1501. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.Y.; Fu, S.Z.; Feng, S.S. Nanohydrogels as a prospective member of the nanomedicine family. Nanomedicine 2013, 8, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.S.; Chiu, D.T. Soft fluorescent nanomaterials for biological and biomedical imaging. Chem. Soc. Rev. 2015, 44, 4699–4722. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhai, Y.; Wang, J.; Zhai, G. New progress and prospects: The application of nanogel in drug delivery. Mater. Sci. Eng. C 2016, 60, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Eckmann, D.M.; Composto, R.J.; Tsourkas, A.; Muzykantov, V.R. Nanogel carrier design for targeted drug delivery. J. Mater. Chem. B 2014, 2, 8085–8097. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, J.; Deng, C.; Suuronen, E.J.; Zhong, Z. Click hydrogels, microgels and nanogels: Emerging platforms for drug delivery and tissue engineering. Biomaterials 2014, 35, 4969–4985. [Google Scholar] [CrossRef]
- Oh, J.K.; Lee, D.I.; Park, J.M. Biopolymer-based microgels/nanogels for drug delivery applications. Prog. Polym. Sci. 2009, 34, 1261–1282. [Google Scholar] [CrossRef]
- Chacko, R.T.; Ventura, J.; Zhuang, J.; Thayumanavan, S. Polymer nanogels: A versatile nanoscopic drug delivery platform. Adv. Drug Deliv. Rev. 2012, 64, 836–851. [Google Scholar] [CrossRef]
- Tang, Z.; He, C.; Tian, H.; Ding, J.; Hsiao, B.S.; Chu, B.; Chen, X. Polymeric nanostructured materials for biomedical applications. Prog. Polym. Sci. 2016, 60, 86–128. [Google Scholar] [CrossRef]
- Kabanov, A.V.; Vinogradov, S.V. Nanogels as pharmaceutical carriers: Finite networks of infinite capabilities. Angew. Chemie Int. Ed. 2009, 48, 5418–5429. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.Q.; Wang, C.C. Biodegradable smart nanogels: A new platform for targeting drug delivery and biomedical diagnostics. Langmuir 2016, 32, 6211–6225. [Google Scholar] [CrossRef] [PubMed]
- Karg, M. Functional Materials Design through Hydrogel Encapsulation of Inorganic Nanoparticles: Recent Developments and Challenges. Macromol. Chem. Phys. 2016, 217, 242–255. [Google Scholar] [CrossRef]
- Molina, M.; Asadian-Birjand, M.; Balach, J.; Bergueiro, J.; Miceli, E.; Calderón, M. Stimuli-responsive nanogel composites and their application in nanomedicine. Chem. Soc. Rev. 2015, 44, 6161–6186. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.; Felidj, N.; Mangeney, C. Looking for Synergies in Molecular Plasmonics through Hybrid Thermoresponsive Nanostructures. Chem. Mater. 2016, 28, 3564–3577. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Q.; Zhou, S. Carbon-based hybrid nanogels: A synergistic nanoplatform for combined biosensing, bioimaging, and responsive drug delivery. Chem. Soc. Rev. 2018, 47, 4198–4232. [Google Scholar] [CrossRef]
- Raemdonck, K.; Braeckmans, K.; Demeester, J.; De Smedt, S.C. Merging the best of both worlds: Hybrid lipid-enveloped matrix nanocomposites in drug delivery. Chem. Soc. Rev. 2014, 43, 444–472. [Google Scholar] [CrossRef]
- Sierra-Martin, B.; Fernandez-Barbero, A. Multifunctional hybrid nanogels for theranostic applications. Soft Matter 2015, 11, 8205–8216. [Google Scholar] [CrossRef]
- Rejinold, N.S.; Jayakumar, R.; Kim, Y.C. Radio frequency responsive nano-biomaterials for cancer therapy. J. Control. Release 2015, 204, 85–97. [Google Scholar] [CrossRef]
- Li, Z.; Ye, E.; David; Lakshminarayanan, R.; Loh, X.J. Recent Advances of Using Hybrid Nanocarriers in Remotely Controlled Therapeutic Delivery. Small 2016, 12, 4782–4806. [Google Scholar] [CrossRef] [PubMed]
- Mai, B.T.; Fernandes, S.; Balakrishnan, P.B.; Pellegrino, T. Nanosystems Based on Magnetic Nanoparticles and Thermo- or pH-Responsive Polymers: An Update and Future Perspectives. Acc. Chem. Res. 2018, 51, 999–1013. [Google Scholar] [CrossRef] [PubMed]
- Macchione, M.A.; Biglione, C.; Strumia, M. Design, synthesis and architectures of hybrid nanomaterials for therapy and diagnosis applications. Polymers (Basel) 2018, 10, 527. [Google Scholar] [CrossRef]
- Kowalczuk, A.; Trzcinska, R.; Trzebicka, B.; Müller, A.H.E.; Dworak, A.; Tsvetanov, C.B. Loading of polymer nanocarriers: Factors, mechanisms and applications Dedicated to Prof. Stanislaw Penczek on the occasion of his 80th birthday. Prog. Polym. Sci. 2014, 39, 43–86. [Google Scholar] [CrossRef]
- Cayre, O.J.; Chagneux, N.; Biggs, S. Stimulus responsive core-shell nanoparticles: Synthesis and applications of polymer based aqueous systems. Soft Matter 2011, 7, 2211–2234. [Google Scholar] [CrossRef]
- Plamper, F.A.; Richtering, W. Functional Microgels and Microgel Systems. Acc. Chem. Res. 2017, 50, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Panday, R.; Poudel, A.J.; Li, X.; Adhikari, M.; Ullah, M.W.; Yang, G. Amphiphilic core-shell nanoparticles: Synthesis, biophysical properties, and applications. Colloids Surf. B Biointerfaces 2018, 172, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, U.; Nomura, S.I.M.; Kaul, S.C.; Hirano, T.; Akiyoshi, K. Nanogel-quantum dot hybrid nanoparticles for live cell imaging. Biochem. Biophys. Res. Commun. 2005, 331, 917–921. [Google Scholar] [CrossRef]
- Liu, J.; Detrembleur, C.; Mornet, S.; Jérôme, C.; Duguet, E. Design of hybrid nanovehicles for remotely triggered drug release: An overview. J. Mater. Chem. B 2015, 3, 6117–6147. [Google Scholar] [CrossRef]
- Desale, S.S.; Cohen, S.M.; Zhao, Y.; Kabanov, A.V.; Bronich, T.K. Biodegradable hybrid polymer micelles for combination drug therapy in ovarian cancer. J. Control. Release 2013, 3, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Ekkelenkamp, A.E.; Elzes, M.R.; Engbersen, J.F.J.; Paulusse, J.M.J. Responsive crosslinked polymer nanogels for imaging and therapeutics delivery. J. Mater. Chem. B 2018, 6, 210–235. [Google Scholar] [CrossRef]
- Hood, M.A.; Mari, M.; Muñoz-Espí, R. Synthetic strategies in the preparation of polymer/inorganic hybrid nanoparticles. Materials (Basel) 2014, 7, 4057–4087. [Google Scholar] [CrossRef] [PubMed]
- Mavila, S.; Eivgi, O.; Berkovich, I.; Lemcoff, N.G. Intramolecular Cross-Linking Methodologies for the Synthesis of Polymer Nanoparticles. Chem. Rev. 2016, 116, 878–961. [Google Scholar] [CrossRef] [PubMed]
- Landfester, K.; Musyanovych, A. Hydrogels in miniemulsions. In Chemical Design of Responsive Microgels; 2010; Volume 234, pp. 39–63. [Google Scholar] [CrossRef]
- Cao, Z.; Ziener, U. Synthesis of nanostructured materials in inverse miniemulsions and their applications. Nanoscale 2013, 5, 10093–10107. [Google Scholar] [CrossRef]
- Oh, J.K.; Bencherif, S.A.; Matyjaszewski, K. Atom transfer radical polymerization in inverse miniemulsion: A versatile route toward preparation and functionalization of microgels/nanogels for targeted drug delivery applications. Polymer (Guildf) 2009, 50, 4407–4423. [Google Scholar] [CrossRef]
- Sanson, N.; Rieger, J. Synthesis of nanogels/microgels by conventional and controlled radical crosslinking copolymerization. Polym. Chem. 2010, 1, 965–977. [Google Scholar] [CrossRef]
- Siegwart, D.J.; Oh, J.K.; Matyjaszewski, K. ATRP in the design of functional materials for biomedical applications. Prog. Polym. Sci. 2012, 37, 18–37. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Wang, F.; Wang, C. Reflux Precipitation Polymerization: A New Platform for the Preparation of Uniform Polymeric Nanogels for Biomedical Applications. Macromol. Biosci. 2018, 18, 1–11. [Google Scholar] [CrossRef]
- Cortez-Lemus, N.A.; Licea-Claverie, A. Poly(N-vinylcaprolactam), a comprehensive review on a thermoresponsive polymer becoming popular. Prog. Polym. Sci. 2016, 53, 1–51. [Google Scholar] [CrossRef]
- Motornov, M.; Roiter, Y.; Tokarev, I.; Minko, S. Stimuli-responsive nanoparticles, nanogels and capsules for integrated multifunctional intelligent systems. Prog. Polym. Sci. 2010, 35, 174–211. [Google Scholar] [CrossRef]
- Seo, M.; Beck, B.J.; Paulusse, J.M.J.; Hawker, C.J.; Kim, S.Y. Polymeric nanoparticles via noncovalent cross-linking of linear chains. Macromolecules 2008, 41, 6413–6418. [Google Scholar] [CrossRef]
- Cao, R.; Gu, Z.; Hsu, L.; Patterson, G.D.; Armitage, B.A. Synthesis and characterization of thermoreversible biopolymer microgels based on hydrogen bonded nucleobase pairing. J. Am. Chem. Soc. 2003, 125, 10250–10256. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ballard, N.; Bon, S.A.F. Waterborne polymer nanogels non-covalently crosslinked by multiple hydrogen bond arrays. Polym. Chem. 2013, 4, 387–392. [Google Scholar] [CrossRef]
- Ramos, J.; Forcada, J.; Hidalgo-Alvarez, R. Cationic Polymer Nanoparticles and Nanogels: From Synthesis to Biotechnological Applications. Chem. Rev. 2014, 114, 367–428. [Google Scholar] [CrossRef] [PubMed]
- Acar, H.; Ting, J.M.; Srivastava, S.; LaBelle, J.L.; Tirrell, M.V. Molecular engineering solutions for therapeutic peptide delivery. Chem. Soc. Rev. 2017, 46, 6553–6569. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Liu, D.; Subramanyam, K.; Wang, B.; Yang, J.; Huang, J.; Auguste, D.T.; Moses, M.A. Nanoparticle elasticity directs tumor uptake. Nat. Commun. 2018, 9, 130. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Akiyoshi, K. Nanogel engineering for new nanobiomaterials: From chaperoning engineering to biomedical applications. Chem. Rec. 2010, 10, 366–376. [Google Scholar] [CrossRef]
- Moshe, H.; Davizon, Y.; Menaker Raskin, M.; Sosnik, A. Novel poly(vinyl alcohol)-based amphiphilic nanogels by non-covalent boric acid crosslinking of polymeric micelles. Biomater. Sci. 2017, 5, 2295–2309. [Google Scholar] [CrossRef]
- Wang, S.; Ha, Y.; Huang, X.; Chin, B.; Sim, W.; Chen, R. A New Strategy for Intestinal Drug Delivery via pH-Responsive and Membrane-Active Nanogels. ACS Appl. Mater. Interfaces 2018, 10, 36622–36627. [Google Scholar] [CrossRef]
- Soni, K.S.; Desale, S.S.; Bronich, T.K. Nanogels: An overview of properties, biomedical applications and obstacles to clinical translation. J. Control. Release 2016, 240, 109–126. [Google Scholar] [CrossRef]
- Bertrand, N.; Grenier, P.; Mahmoudi, M.; Lima, E.M.; Appel, E.A.; Dormont, F.; Lim, J.M.; Karnik, R.; Langer, R.; Farokhzad, O.C. Mechanistic understanding of in vivo protein corona formation on polymeric nanoparticles and impact on pharmacokinetics. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Vicario-de-la-Torre, M.; Forcada, J. The Potential of Stimuli-Responsive Nanogels in Drug and Active Molecule Delivery for Targeted Therapy. Gels 2017, 3, 16. [Google Scholar] [CrossRef]
- Allen, T.M. Drug Delivery Systems: Entering the Mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef]
- Brigger, I.; Dubernet, C.; Couvreur, P. Nanoparticles in cancer therapy and diagnosis. Adv. Drug Deliv. Rev. 2002, 54, 631–651. [Google Scholar] [CrossRef]
- Gamucci, O.; Bertero, A.; Gagliardi, M.; Bardi, G. Biomedical Nanoparticles: Overview of Their Surface Immune-Compatibility. Coatings 2014, 4, 139–159. [Google Scholar] [CrossRef]
- Lima, A.C.; Alvarez-Lorenzo, C.; Mano, J.F. Design Advances in Particulate Systems for Biomedical Applications. Adv. Healthc. Mater. 2016, 5, 1687–1723. [Google Scholar] [CrossRef] [PubMed]
- Ke, P.C.; Lin, S.; Parak, W.J.; Davis, T.P.; Caruso, F. A Decade of the Protein Corona. ACS Nano 2017, 11, 11773–11776. [Google Scholar] [CrossRef] [PubMed]
- Monopoli, M.P.; Åberg, C.; Salvati, A.; Dawson, K.A. Biomolecular coronas provide the biological identity of nanosized materials. Nat. Nanotechnol. 2012, 7, 779–786. [Google Scholar] [CrossRef]
- Treuel, L.; Nienhaus, G.U. Toward a molecular understanding of nanoparticle-protein interactions. Biophys. Rev. 2012, 4, 137–147. [Google Scholar] [CrossRef]
- Hu, C.-M.J.; Zhang, L.; Aryal, S.; Cheung, C.; Fang, R.H.; Zhang, L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl. Acad. Sci. USA 2011, 108, 10980–10985. [Google Scholar] [CrossRef]
- Longmire, M.; Choyke, P.L.; Kobayashi, H. Clearance properties of nano-sized particles and molecules as imaging agents: considerations and caveats. Nanomedicine 2008, 3, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Tenzer, S.; Docter, D.; Rosfa, S.; Wlodarski, A.; Rekik, A.; Knauer, S.K.; Bantz, C.; Nawroth, T.; Bier, C.; Sirirattanapan, J.; et al. Nanoparticle Size Is a Critical Physico- chemicalDeterminantoftheHumanBlood Plasma Corona: A Comprehensive Quantitative Proteomic Analysis. ACS Nano 2011, 5, 7155–7167. [Google Scholar] [CrossRef] [PubMed]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors Affecting the Clearance and Biodistribution of Polymeric Nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Papasani, M.R.; Wang, G.; Hill, R.A. Gold nanoparticles: The importance of physiological principles to devise strategies for targeted drug delivery. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Zheng, J. Clearance Pathways and Tumor Targeting of Imaging Nanoparticles. ACS Nano 2015, 9, 6655–6674. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, H.H.; Holt-Casper, D.; Grainger, D.W.; Ghandehari, H. Nanoparticle uptake: The phagocyte problem. Nano Today 2015, 10, 487–510. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Wilhelm, S.; Ding, D.; Syed, A.M.; Sindhwani, S.; Zhang, Y.; Chen, Y.Y.; Macmillan, P.; Chan, W.C.W. Quantifying the Ligand-Coated Nanoparticle Delivery to Cancer Cells in Solid Tumours. ACS Nano 2018. [Google Scholar] [CrossRef]
- Du, B.; Yu, M.; Zheng, J. Transport and interactions of nanoparticles in the kidneys. Nat. Rev. Mater. 2018, 3. [Google Scholar] [CrossRef]
- Veiseh, O.; Gunn, J.W.; Zhang, M. Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Adv. Drug Deliv. Rev. 2010, 62, 284–304. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Xu, F.; Yuan, Y.; Shan, X.; Liu, C.; Tao, X.; Sheng, Y.; Zhou, H. Long-circulation of hemoglobin-loaded polymeric nanoparticles as oxygen carriers with modulated surface charges. Int. J. Pharm. 2009, 377, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Papi, M.; Caputo, D.; Palmieri, V.; Coppola, R.; Palchetti, S.; Bugli, F.; Martini, C.; Digiacomo, L.; Pozzi, D.; Caracciolo, G. Clinically approved PEGylated nanoparticles are covered by a protein corona that boosts the uptake by cancer cells. Nanoscale 2017, 9, 10327–10334. [Google Scholar] [CrossRef] [PubMed]
- Oishi, M.; Tamura, A.; Nakamura, T.; Nagasaki, Y. A smart nanoprobe based on fluorescence-quenching PEGylated nanogels containing gold nanopartlcles for monitoring the response to cancer therapy. Adv. Funct. Mater. 2009, 19, 827–834. [Google Scholar] [CrossRef]
- Nakamura, T.; Tamura, A.; Murotani, H.; Oishi, M.; Jinji, Y.; Matsuishi, K.; Nagasaki, Y. Large payloads of gold nanoparticles into the polyamine network core of stimuli-responsive PEGylated nanogels for selective and noninvasive cancer photothermal therapy. Nanoscale 2010, 2, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Manzanares-Guevara, L.A.; Licea-Claverie, A.; Paraguay-Delgado, F. Preparation of stimuli-responsive nanogels based on poly(N,N-diethylaminoethyl methacrylate) by a simple “surfactant-free” methodology. Soft Mater. 2018, 16, 37–50. [Google Scholar] [CrossRef]
- Wu, W.; Shen, J.; Banerjee, P.; Zhou, S. Water-dispersible multifunctional hybrid nanogels for combined curcumin and photothermal therapy. Biomaterials 2011, 32, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Di, J.; Sun, Y.; Fu, J.; Wei, Z.; Matsui, H.; del C. Alonso, A.; Zhou, S. Biocompatible PEG-Chitosan@Carbon Dots Hybrid Nanogels for Two-Photon Fluorescence Imaging, Near-Infrared Light/pH Dual-Responsive Drug Carrier, and Synergistic Therapy. Adv. Funct. Mater. 2015, 25, 5537–5547. [Google Scholar] [CrossRef]
- Park, J.; Wrzesinski, S.H.; Stern, E.; Look, M.; Criscione, J.; Ragheb, R.; Jay, S.M.; Demento, S.L.; Agawu, A.; Licona Limon, P.; et al. Combination delivery of TGF-β inhibitor and IL-2 by nanoscale liposomal polymeric gels enhances tumour immunotherapy. Nat. Mater. 2012, 11, 895–905. [Google Scholar] [CrossRef]
- Look, M.; Stern, E.; Wang, Q.A.; DiPlacido, L.D.; Kashgarian, M.; Craft, J.; Fahmy, T.M. Nanogel-based delivery of mycophenolic acid ameliorates systemic lupus erythematosus in mice. J. Clin. Investig. 2013, 123, 1741–1749. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Lee, J.S.; Choi, J.H.; Lee, Y.; Son, J.Y.; Bae, J.W.; Lee, K.; Park, K.D. Heparin nanogel-containing liposomes for intracellular RNase delivery. Macromol. Res. 2015, 23, 765–769. [Google Scholar] [CrossRef]
- Yu, L.; Dong, A.; Guo, R.; Yang, M.; Deng, L.; Zhang, J. DOX/ICG Coencapsulated Liposome-Coated Thermosensitive Nanogels for NIR-Triggered Simultaneous Drug Release and Photothermal Effect. ACS Biomater. Sci. Eng. 2018, 4, 2424–2434. [Google Scholar] [CrossRef]
- Zhang, L.; Xue, H.; Cao, Z.; Keefe, A.; Wang, J.; Jiang, S. Multifunctional and degradable zwitterionic nanogels for targeted delivery, enhanced MR imaging, reduction-sensitive drug release, and renal clearance. Biomaterials 2011, 32, 4604–4608. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cao, Z.; Li, Y.; Ella-Menye, J.R.; Bai, T.; Jiang, S. Softer zwitterionic nanogels for longer circulation and lower splenic accumulation. ACS Nano 2012, 6, 6681–6686. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, Q.; Zhang, P.; Liu, L. Zwitterionic nanogels crosslinked by fluorescent carbon dots for targeted drug delivery and simultaneous bioimaging. Acta Biomater. 2016, 40, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Barenholz, Y. Doxil® - The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Turecek, P.L.; Bossard, M.J.; Schoetens, F.; Ivens, I.A. PEGylation of Biopharmaceuticals: A Review of Chemistry and Nonclinical Safety Information of Approved Drugs. J. Pharm. Sci. 2016, 105, 460–475. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.M.; Goyal, B.R.; Bhadada, S.V.; Bhatt, J.S.; Amin, A.F. Getting into the Brain. CNS Drugs 2009, 23, 35–58. [Google Scholar] [CrossRef]
- Motlaq, V.F.; Knudsen, K.D.; Nyström, B. Effect of PEGylation on the stability of thermoresponsive nanogels. J. Colloid Interface Sci. 2018, 524, 245–255. [Google Scholar] [CrossRef]
- Amoozgar, Z.; Yeo, Y. Recent advances in stealth coating of nanoparticle drug delivery systems. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2012, 4, 219–233. [Google Scholar] [CrossRef]
- Koshkaryev, A.; Sawant, R.; Deshpande, M.; Torchilin, V. Immunoconjugates and long circulating systems: Origins, current state of the art and future directions. Adv. Drug Deliv. Rev. 2013, 65, 24–35. [Google Scholar] [CrossRef]
- Sugiyama, I.; Sadzuka, Y. Change in the Character of Liposomes as a Drug Carrier by Modifying Various Polyethyleneglycol-Lipids. Biol. Pharm. Bull. 2013, 900, 900–906. [Google Scholar] [CrossRef]
- Gref, R.; Lück, M.; Quellec, P.; Marchand, M.; Dellacherie, E.; Harnisch, S.; Blunk, T.; Müller, R.H. “Stealth” corona-core nanoparticles surface modified by polyethylene glycol (PEG): Influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption. Colloids Surf. B Biointerfaces 2000, 18, 301–313. [Google Scholar] [CrossRef]
- Jokerst, J.V.; Lobovkina, T.; Zare, R.N.; Gambhir, S.S. Nanoparticle PEGylation for imaging and therapy. Nanomedicine 2011, 6, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [PubMed]
- Mauri, E.; Cappella, F.; Masi, M.; Rossi, F. PEGylation influences drug delivery from nanogels. J. Drug Deliv. Sci. Technol. 2018, 46, 87–92. [Google Scholar] [CrossRef]
- Li, S.D.; Huang, L. Stealth nanoparticles: High density but sheddable PEG is a key for tumor targeting. J. Control. Release 2010, 145, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Lai, S.K. Anti-PEG immunity: Emergence, characteristics, and unaddressed questions. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 655–677. [Google Scholar] [CrossRef]
- Oishi, M.; Hayashi, H.; Iijima, M.; Nagasaki, Y. Endosomal release and intracellular delivery of anticancer drugs using pH-sensitive PEGylated nanogels. J. Mater. Chem. 2007, 17, 3720. [Google Scholar] [CrossRef]
- Oishi, M.; Hayashi, H.; Uno, T.; Ishii, T.; Iijima, M.; Nagasaki, Y. One-Pot Synthesis of pH-Responsive PEGylated Nanogels Containing Gold Nanoparticles by Autoreduction of Chloroaurate Ions within Nanoreactors. Macromol. Chem. Phys. 2007, 208, 1176–1182. [Google Scholar] [CrossRef]
- Oishi, M.; Hayashi, H.; Itaka, K.; Kataoka, K.; Nagasaki, Y. pH-Responsive PEGylated nanogels as targetable and low invasive endosomolytic agents to induce the enhanced transfection efficiency of nonviral gene vectors. Colloid Polym. Sci. 2007, 285, 1055–1060. [Google Scholar] [CrossRef]
- Hossain, M.A.; Ikeda, Y.; Hara, T.; Nagasaki, Y. Novel biocompatible nanoreactor for silica/gold hybrid nanoparticles preparation. Colloids Surf. B Biointerfaces 2013, 102, 778–782. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Ikeda, Y.; Nagasaki, Y. PEGylated polyamine nanogel as a nanoreactor of silica/Gold hybrid nanoparticle preparation. J. Photopolym. Sci. Technol. 2011, 24, 603–606. [Google Scholar] [CrossRef]
- Yasui, H.; Takeuchi, R.; Nagane, M.; Meike, S.; Nakamura, Y.; Yamamori, T.; Ikenaka, Y.; Kon, Y.; Murotani, H.; Oishi, M.; et al. Radiosensitization of tumor cells through endoplasmic reticulum stress induced by PEGylated nanogel containing gold nanoparticles. Cancer Lett. 2014, 347, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Pabla, N.; Dong, Z. Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int. 2008, 73, 994–1007. [Google Scholar] [CrossRef] [PubMed]
- Boyman, O.; Sprent, J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat. Rev. Immunol. 2012, 12, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tsokos, M.G.; Bickerton, S.; Sharabi, A.; Li, Y.; Moulton, V.R.; Kong, P.; Fahmy, T.M.; Tsokos, G.C. Precision DNA demethylation ameliorates disease in lupus-prone mice. JCI Insight 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Deng, L.; Deng, H.; Dong, A.; Wang, W.; Zhang, J. In Situ Template Polymerization to Prepare Liposome-Coated PDMAEMA Nanogels with Controlled Size, High Stability, Low Cytotoxicity, and Responsive Drug Release for Intracellular DOX Release. Macromol. Chem. Phys. 2018, 219, 1–12. [Google Scholar] [CrossRef]
- Ma, J.; Deng, H.; Zhao, F.; Deng, L.; Wang, W.; Dong, A.; Zhang, J. Liposomes-Camouflaged Redox-Responsive Nanogels to Resolve the Dilemma between Extracellular Stability and Intracellular Drug Release. Macromol. Biosci. 2018, 18, 1–10. [Google Scholar] [CrossRef]
- Cao, Z.Q.; Jiang, S.Y. Super-hydrophilic zwitterionic poly(carboxybetaine) and amphiphilic non-ionic poly(ethylene glycol) for stealth nanoparticles. Nano Today 2012, 7, 404–413. [Google Scholar] [CrossRef]
- Schlenoff, J.B. Zwitteration: Coating surfaces with zwitterionic functionality to reduce nonspecific adsorption. Langmuir 2014, 30, 9625–9636. [Google Scholar] [CrossRef]
- García, K.P.; Zarschler, K.; Barbaro, L.; Barreto, J.A.; O’Malley, W.; Spiccia, L.; Stephan, H.; Graham, B. Zwitterionic-coated “stealth” nanoparticles for biomedical applications: Recent advances in countering biomolecular corona formation and uptake by the mononuclear phagocyte system. Small 2014, 10, 2516–2529. [Google Scholar] [CrossRef] [PubMed]
- Lynch, I.; Dawson, K.A. Protein-nanoparticle interactions. Nano Today 2008, 3, 40–47. [Google Scholar] [CrossRef]
- Banquy, X.; Suarez, F.; Argaw, A.; Rabanel, J.-M.; Grutter, P.; Bouchard, J.-F.; Hildgen, P.; Giasson, S. Effect of mechanical properties of hydrogel nanoparticles on macrophage cell uptake. Soft Matter 2009, 5, 3984. [Google Scholar] [CrossRef]
- Mebius, R.E.; Kraal, G. Structure and function of the spleen. Nat. Rev. Immunol. 2005, 5, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Merkel, T.J.; Jones, S.W.; Herlihy, K.P.; Kersey, F.R.; Shields, A.R.; Napier, M.; Luft, J.C.; Wu, H.; Zamboni, W.C.; Wang, A.Z.; et al. Using mechanobiological mimicry of red blood cells to extend circulation times of hydrogel microparticles. Proc. Natl. Acad. Sci. USA 2011, 108, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, W.; Singh, A.; Cheng, G.; Liu, L. Two amino acid-based superlow fouling polymers: Poly(lysine methacrylamide) and poly(ornithine methacrylamide). Acta Biomater. 2014, 10, 2956–2964. [Google Scholar] [CrossRef] [PubMed]
- Mourran, A.; Wu, Y.; Gumerov, R.A.; Rudov, A.A.; Potemkin, I.I.; Pich, A.; Möller, M. When Colloidal Particles Become Polymer Coils. Langmuir 2016, 32, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.B.; Zhang, F.; Wang, C.C. Rational synthesis of magnetic thermosensitive microcontainers as targeting drug carriers. Small 2009, 5, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Kettel, M.J.; Schaefer, K.; Pich, A.; Moeller, M. Functional PMMA nanogels by cross-linking with cyclodextrin methacrylate. Polymer (Guildf) 2016, 86, 176–188. [Google Scholar] [CrossRef]
- Danhier, F.; Feron, O.; Préat, V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control. Release 2010, 148, 135–146. [Google Scholar] [CrossRef]
- Desai, N. Challenges in Development of Nanoparticle-Based Therapeutics. AAPS J. 2012, 14, 282–295. [Google Scholar] [CrossRef] [PubMed]
- Park, K. Facing the truth about nanotechnology in drug delivery. ACS Nano 2013, 7, 7442–7447. [Google Scholar] [CrossRef]
- Kitano, S.; Kageyama, S.; Nagata, Y.; Miyahara, Y.; Hiasa, A.; Naota, H.; Okumura, S.; Imai, H.; Shiraishi, T.; Masuya, M.; et al. HER2-specific T-cell immune responses in patients vaccinated with truncated HER2 protein complexed with nanogels of cholesteryl pullulan. Clin. Cancer Res. 2006, 12, 7397–7405. [Google Scholar] [CrossRef] [PubMed]
- Kyogoku, N.; Ikeda, H.; Tsuchikawa, T.; Abiko, T.; Fujiwara, A.; Maki, T.; Yamamura, Y.; Ichinokawa, M.; Tanaka, K.; Imai, N.; et al. Time-dependent transition of the immunoglobulin g subclass and immunoglobulin E response in cancer patients vaccinated with cholesteryl pullulan-melanoma antigen gene-a4 nanogel. Oncol. Lett. 2016, 12, 4493–4504. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Wada, H.; Yamasaki, M.; Miyata, H.; Nishikawa, H.; Sato, E.; Kageyama, S.; Shiku, H.; Mori, M.; Doki, Y. High expression of MAGE-A4 and MHC class I antigens in tumor cells and induction of MAGE-A4 immune responses are prognostic markers of CHP-MAGE-A4 cancer vaccine. Vaccine 2014, 32, 5901–5907. [Google Scholar] [CrossRef] [PubMed]
- Uenaka, A.; Wada, H.; Isobe, M.; Saika, T.; Tsuji, K.; Sato, E.; Sato, S.; Noguchi, Y.; Kawabata, R.; Yasuda, T.; et al. T cell immunomonitoring and tumor responses in patients immunized with a complex of cholesterol-bearing hydrophobized pullulan (CHP) and NY-ESO-1 protein. Cancer Immun. 2007, 7, 9. [Google Scholar]
- Kageyama, S.; Kitano, S.; Hirayama, M.; Nagata, Y.; Imai, H.; Shiraishi, T.; Akiyoshi, K.; Scott, A.M.; Murphy, R.; Hoffman, E.W.; et al. Humoral immune responses in patients vaccinated with 1-146 HER2 protein complexed with cholesteryl pullulan nanogel. Cancer Sci. 2008, 29, 601–607. [Google Scholar] [CrossRef]
- Barua, S.; Mitragotri, S. Challenges associated with penetration of nanoparticles across cell and tissue barriers: A review of current status and future prospects. Nano Today 2014, 9, 223–243. [Google Scholar] [CrossRef]
- Vlastou, E.; Gazouli, M.; Ploussi, A.; Platoni, K.; Efstathopoulos, E.P. Nanoparticles: nanotoxicity aspects. J. Phys. Conf. Ser. 2017, 931, 012020. [Google Scholar] [CrossRef]
- Khanna, P.; Ong, C.; Bay, B.; Baeg, G. Nanotoxicity: An Interplay of Oxidative Stress, Inflammation and Cell Death. Nanomaterials 2015, 5, 1163–1180. [Google Scholar] [CrossRef]
- Tipnis, N.P.; Burgess, D.J. Sterilization of implantable polymer-based medical devices: A review. Int. J. Pharm. 2018, 544, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Cingolani, A.; Casalini, T.; Caimi, S.; Klaue, A.; Sponchioni, M.; Rossi, F.; Perale, G. A methodologic approach for the selection of bio-resorbable polymers in the development of medical devices: The case of poly(L-lactide-co-ε-caprolactone). Polymers (Basel) 2018, 10, 851. [Google Scholar] [CrossRef]















| Schematized Structure of the Hybrid | Stealth Strategy | Size | Biological Results | Reference |
|---|---|---|---|---|
 | PEG chains (~2400 Da, 7200 Da 1) | 80 nm a | In vitro: no toxicity on HuH-7 (50 μg/mL), HeLa (480 µg/mL) 1 | [74,75] |
 | PEG shell (500–4000 Da) | 52–350 nm a | (see note 2) | [76] |
 | PEG gel (300 Da), PEG shell (550 Da) | 25–40 nm a [40–60 b] | In vitro: no toxicity on B16F10 mouse melanoma cells (450 μg/mL) | [77] |
 | PEG/chitosan gel, PEG chains (550 Da) | 120 nm a (pH 7.4) [180 nm b] | In vitro: no toxicity on DU145 cells (100 μg/mL) In vivo: C57BL/6 mice models, histology: no signs of toxicity on liver and kidney. | [78] |
 | PEG shell (5000 Da) | 90 nm a (pH 7.4) | In vitro: no toxicity on A2780 cells (5 mg/mL) In vivo: xenograft mice, histology: no alteration on kidney tissue, no signs of splenic or hepatic toxicity, most platinum accumulated eliminated after 1 month. | [30] |
 | PEGylated liposome (2000 Da), bioresorbable gel | 120 nm a | In vitro: only release tests in phosphate buffer (pH 7.4) In vivo: B16/B6 mice models: absence of renal and hepatic toxicity. Blood values in physiological ranges, no signs of pulmonary toxicity. No inflammatory response markers. Extended circulation lifetime of carried drug, improved biodistribution. | [79] |
 | PEGylated liposome (2000 Da), bioresorbable gel | 225 nm a | In vitro: only internalization tests on CD4 T cells In vivo: C57BL/6 mice models: no hepatic, hematological and general organ toxicity; repeated doses and analyses show values in physiological ranges: complete blood counts, renal and hepatic functions. Protect from nephritis. | [80] |
 | PEGylated liposome (4000 Da), biocompatible gel | 300 nm a | In vitro: no toxicity on NIH3T3 mouse fibroblasts (100 μg/mL) | [81] |
 | PEGylated liposome (2000 Da) | 106 nm a [103 nm b] | In vitro: no toxicity on 4T1 murine cancer cells (1000 μg/mL), hemocompatibility on human blood (100 μg/mL) | [82] |
 | Zwitterionic polymer: carboxybetaine, reduction-sensitive crosslinker | 110 nm a | In vitro: no cytotoxicity on macrophages (RAW264.7) and HUVEC cells (iron content: 30 ppm) 3 | [83] |
 | Zwitterionic polymer: carboxybetaine, softness of the structure | 120 nm a | In vitro: no uptake from macrophage cells (5 ppm of Au, corresponding to 42 μg/mL of nanogel) In vivo: Sprague Dawley rats: biodistribution study, most accumulation in liver and spleen. Increasing softness extend the circulation half-life and reduces splenic accumulation | [84] |
 | Zwitterionic polymer: ornithine | 114 nm a | In vitro: 90% viability on NIH/3T3 fibroblasts (1000 μg/mL), no protein bonded after incubation in protein solution | [85] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eslami, P.; Rossi, F.; Fedeli, S. Hybrid Nanogels: Stealth and Biocompatible Structures for Drug Delivery Applications. Pharmaceutics 2019, 11, 71. https://doi.org/10.3390/pharmaceutics11020071
Eslami P, Rossi F, Fedeli S. Hybrid Nanogels: Stealth and Biocompatible Structures for Drug Delivery Applications. Pharmaceutics. 2019; 11(2):71. https://doi.org/10.3390/pharmaceutics11020071
Chicago/Turabian StyleEslami, Parisa, Filippo Rossi, and Stefano Fedeli. 2019. "Hybrid Nanogels: Stealth and Biocompatible Structures for Drug Delivery Applications" Pharmaceutics 11, no. 2: 71. https://doi.org/10.3390/pharmaceutics11020071