Development of a Resveratrol Nanosuspension Using the Antisolvent Precipitation Method without Solvent Removal, Based on a Quality by Design (QbD) Approach
Abstract
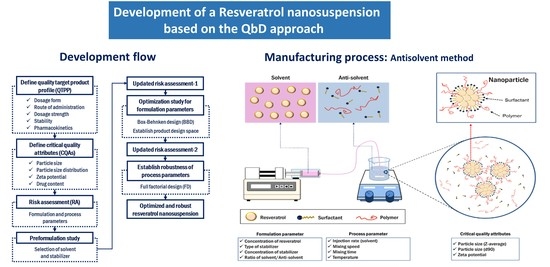
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Definition of the Quality Target Product Profile and Critical Quality Attributes
2.3. Initial Risk Assessment for Formulation and Process Parameters
2.4. Preformulation of the Resveratrol Nanosuspension
2.4.1. Solubility of Resveratrol in Various Solvents
2.4.2. Inhibitory Effect of Polymers on Resveratrol Precipitation
2.5. Preparation of Resveratrol Nanosuspensions
2.6. Design of Experiments for the Preparation of Resveratrol Nanosuspensions
2.6.1. Box–Behnken Design for the Optimization of Formulation Parameters
2.6.2. Full Factorial Design to Establish the Robustness of the Process Parameters
2.7. Characterization of the Resveratrol Nanosuspension
2.7.1. Measurement of Drug Content (%)
2.7.2. Measurement of Particle Size and Zeta Potential
2.7.3. Morphological Characterization
2.7.4. In Vitro Dissolution Study
2.8. Pharmacokinetic Study in Rats
2.9. Stability Study
2.10. Statistical Analysis
3. Results and Discussion
3.1. Preformulation of the Resveratrol Nanosuspension
3.1.1. Solubility of Resveratrol in Solvents
3.1.2. Inhibitory Effect of Stabilizers on Resveratrol Precipitation
3.1.3. Screening for Stabilizers
3.2. Optimization Study of the Resveratrol Nanosuspension
3.2.1. Box–Behnken Design for the Optimization of Formulation Parameters
3.2.2. Establishment of the Design Space
3.2.3. Robustness of Process Parameters in the Optimized Formulation
3.3. Characterization of the Optimized Resveratrol Nanosuspension
3.3.1. Dissolution Characterization
3.3.2. Pharmacokinetics in Rats
3.3.3. Long-Term Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AUC0–12 h | the area under the plasma concentration versus time curve calculated using the linear trapezoidal method |
| BCS | Biopharmaceutical Classification System |
| Cmax | the maximum plasma concentration of trans-resveratrol |
| CPP | critical process parameter |
| Cs | saturated solubility |
| CQA | critical quality attribute |
| CV | coefficient of variation |
| d90 | the particle size at 90% cumulative volume percentage |
| DEGEE | diethylene glycol monoethyl ether |
| DLS | dynamic light scattering |
| HPC | hydroxypropyl cellulose |
| HPLC | high-performance liquid chromatography |
| HPMC | hydroxypropyl methyl cellulose |
| ICH | International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use |
| PRESS | predicted residual error sum of squares |
| PVP | polyvinylpyrrolidone |
| PVP VA | polyvinylpyrrolidone vinyl acetate |
| R2 | coefficient of determination |
| RA | risk assessment |
| RC | regenerated cellulose |
| SD | Sprague–Dawley |
| SLS | sodium lauryl sulfate |
| TEM | transmission electron microscope |
| Tmax | the time required to reach Cmax |
| USP | United States Pharmacopeia |
| QbD | quality by design |
| QTPP | quality target product profile |
References
- Singh, G.; Pai, R.S. Recent advances of resveratrol in nanostructured based delivery systems and in the management of HIV/AIDS. J. Controll. Release 2014, 194, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Amri, A.; Chaumeil, J.C.; Sfar, S.; Charrueau, C. Administration of resveratrol: What formulation solutions to bioavailability limitations? J. Controll. Release 2012, 158, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.; Kroon, P.A.; Das, D.K.; Das, S.; Tosaki, A.; Chan, V.; Singer, M.V.; Feick, P. The biological responses to resveratrol and other polyphenols from alcoholic beverages. Alcohol. Clin. Exp. Res. 2009, 33, 1513–1523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musazzi, U.M.; Youm, I.; Murowchick, J.B.; Ezoulin, M.J.; Youan, B.B.C. Resveratrol-loaded nanocarriers: Formulation, optimization, characterization and in vitro toxicity on cochlear cells. Colloids Surf. B Biointerfaces 2014, 118, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.H.; Lee, J.H.; Park, J.W.; Quach, C.H.T.; Moon, S.H.; Cho, Y.S.; Lee, K.H. Resveratrol-loaded polymeric nanoparticles suppress glucose metabolism and tumor growth in vitro and in vivo. Int. J. Pharm. 2015, 478, 251–257. [Google Scholar] [CrossRef]
- Jose, S.; Anju, S.S.; Cinu, T.A.; Aleykutty, N.A.; Thomas, S.; Souto, E.B. In vivo pharmacokinetics and biodistribution of resveratrol-loaded solid lipid nanoparticles for brain delivery. Int. J. Pharm. 2014, 474, 6–13. [Google Scholar] [CrossRef]
- Pandita, D.; Kumar, S.; Poonia, N.; Lather, V. Solid lipid nanoparticles enhance oral bioavailability of resveratrol, a natural polyphenol. Food Res. Int. 2014, 62, 1165–1174. [Google Scholar] [CrossRef]
- Pund, S.; Thakur, R.; More, U.; Joshi, A. Lipid based nanoemulsifying resveratrol for improved physicochemical characteristics, in vitro cytotoxicity and in vivo antiangiogenic efficacy. Colloids Surf. B Biointerfaces 2014, 120, 110–117. [Google Scholar] [CrossRef]
- Amri, A.; Clanche, S.L.; Therond, P.; Bonnefont-Rousselot, D.; Borderie, D.; Lai-Kuen, R.; Chaumeil, J.C.; Sfar, S.; Charrueau, C. Resveratrol self-emulsifying system increases the uptake by endothelial cells and improves protection against oxidative stress-mediated death. Eur. J. Pharm. Biopharm. 2014, 86, 418–426. [Google Scholar] [CrossRef]
- Pangeni, R.; Sharma, S.; Mustafa, G.; Ali, J.; Baboota, S. Vitamin E loaded resveratrol nanoemulsion for brain targeting for the treatment of Parkinson’s disease by reducing oxidative stress. Nanotechnology. 2014, 25, 485102. [Google Scholar] [CrossRef]
- Zhou, J.; Zhou, M.; Yang, F.F.; Liu, C.Y.; Pan, R.L.; Chang, Q.; Liu, X.M.; Liao, Y.H. Involvement of the inhibition of intestinal glucuronidation in enhancing the oral bioavailability of resveratrol by labrasol containing nanoemulsions. Mol. Pharm. 2015, 12, 1084–1095. [Google Scholar] [CrossRef] [PubMed]
- Caddeo, C.; Manconi, M.; Fadda, A.M.; Lai, F.; Lampis, S.; Diez-Sales, O.; Sinico, C. Nanocarriers for antioxidant resveratrol: Formulation approach, vesicle self-assembly and stability evaluation. Colloids Surf. B Biointerfaces 2013, 111, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Isailovic, B.D.; Kostic, I.T.; Zvonar, A.; Dordevic, V.B.; Gasperlin, M.; Nedovic, V.A.; Bugarski, B.M. Resveratrol loaded liposomes produced by different techniques. Innov. Food Sci. Emerg. Technol. 2013, 19, 181–189. [Google Scholar] [CrossRef]
- Andishmand, H.; Tabibiazar, M.; Mohammadifar, M.A.; Hamishehkar, H. Pectin-zinc-chitosan-polyethylene glycol colloidal nano-suspension as a food grade carrier for colon targeted delivery of resveratrol. Int. J. Biol. Macromol. 2017, 97, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Zhao, J.; Zhang, S.; Tong, T.; Zhuang, Q.; Jin, K.; Chen, W.; Tang, H. Fabrication of an ionic-sensitive in situ gel loaded with resveratrol nanosuspensions intended for direct nose-to-brain delivery. Colloids Surf. B Biointerfaces 2016, 147, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Zupancic, S.; Lavric, Z.; Kristl, J. Stability and solubility of trans-resveratrol are strongly influenced by pH and temperature. Eur. J. Pharm. Biopharm. 2015, 93, 196–204. [Google Scholar] [CrossRef]
- Li, J.; Qiao, Y.; Wu, Z. Nanosystem trends in drug delivery using quality-by-design concept. J. Controll. Release 2017, 256, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Lan, Y.; Gokhale, R.; Burgess, D.J. Quality by design approach to understand the process of nanosuspension preparation. Int. J. Pharm. 2009, 377, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.S.M.; York, P.; Blagden, N. Preparation of hydrocortisone nanosuspension through a bottom-up nanoprecipitation technique using microfluidic reactors. Int. J. Pharm. 2009, 375, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, A.M.; Carvalho, F.C.; Teixeria, D.A.; Azevedo, D.L.; de Barros, W.M.; Gremiao, M.P.D. Computational and experimental approaches for development of methotrexate nanosuspensions by bottom-up nanoprecipitation. Int. J. Pharm. 2017, 524, 330–338. [Google Scholar] [CrossRef]
- Salazar, J.; Ghanem, A.; Muller, R.H.; Moschwitzer, J.P. Nanocrystal: Comparison of the size reduction effectiveness of a novel combinative method with conventional top-down approaches. Eur. J. Pharm. Biopharm. 2012, 81, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.X.; Amidon, G.; Khan, M.A.; Hoag, S.W.; Polli, J.; Raju, G.K.; Woodcock, J. Understanding pharmaceutical quality by design. AAPS J. 2014, 16, 771–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ICH Guideline. Pharmaceutical Development Q8 (R2), Step 4 Version; ICH: Geneva, Switzerland, August 2009. [Google Scholar]
- Patravale, V.B.; Date, A.A.; Kulkarni, R.M. Nanosuspension: A promising drug delivery strategy. J. Pharm. Pharmacol. 2004, 56, 827–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Galbraith, S.C.; Ricart, B.; Stanton, C.; Smith-Goettler, B.; Verdi, L.; O’Connor, T.; Lee, S.; Yoon, S. Optimization of critical quality attributes in continuous twin-screw wet granulation via design space validated with pilot scale experimental data. Int. J. Pharm. 2017, 525, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Chin, W.W.L.; Parmentier, J.; Widzinski, M.; Tan, E.H.; Gokhale, R. A brief literature and patent review of nanosuspensions to a final drug product. J. Pharm. Sci. 2014, 103, 2980–2999. [Google Scholar] [CrossRef]
- Das, S.; Ng, K.Y. Quantification of trans-resveratrol in rat plasma by a simple and sensitive high performance liquid chromatography method and its application in pre-clinical study. J. Liq. Chromatogr. Relat. Technol. 2011, 34, 1399–1414. [Google Scholar] [CrossRef]
- Sullivan, Jr.; Dexter, W.; Gad, S.C.; Julien, M. A review of the nonclinical safety of Transcutol®, a highly purified form of diethylene glycol monoethyl ether (DEGEE) used as a pharmaceutical excipient. Food Chem. Toxicol. 2014, 72, 40–50. [Google Scholar] [CrossRef]
- Ha, E.-S.; Lee, S.-K.; Choi, D.H.; Jeong, S.H.; Sung, J.-H.; Kim, M.-S. Application of diethylene glycol monoethyl ether in solubilization of poorly water-soluble drugs. J. Pharm. Investig. 2019. [Google Scholar] [CrossRef]
- Ha, E.-S.; Kuk, D.-H.; Kim, J.-S.; Kim, M.S. Solubility of trans-resveratrol in transcutol HP + water mixtures at different temperatures and its application to fabrication of nanosuspensions. J. Mol. Liq. 2019, 281, 344–351. [Google Scholar] [CrossRef]
- Raghavan, S.L.; Trividic, A.; Davis, A.F.; Hadgraft, J. Crystallization of hydrocortisone acetate: Influence of polymers. Int. J. Pharm. 2001, 212, 213–221. [Google Scholar] [CrossRef]
- Thorat, A.A.; Dalvi, S.V. Liquid antisolvent precipitation and stabilization of nanoparticles of poorly water soluble drugs in aqueous suspensions: Recent developments and future perspective. Chem. Eng. J. 2012, 181, 1–34. [Google Scholar] [CrossRef]
- Kind, M. Colloidal aspects of precipitation processes. Chem. Eng. Sci. 2002, 57, 4287–4293. [Google Scholar] [CrossRef]
- Dalvi, S.V.; Dave, R.N. Controlling particle size of a poorly water-soluble drug using ultrasound and stabilizers in antisolvent precipitation. Ind. Eng. Chem. Res. 2009, 48, 7581–7593. [Google Scholar] [CrossRef]
- Singh, D.; Bedi, N.; Tiwary, A.K. Enhancing solubility of poorly aqueous soluble drugs: Critical appraisal of techniques. J. Pharm. Investig. 2018, 48, 509–526. [Google Scholar] [CrossRef]
- Zimmermann, E.; Muller, R.H. Electrolyte- and pH-stabilities of aqueous solid lipid nanoparticle (SLNTM) dispersions in artificial gastrointestinal media. Eur. J. Pharm. Biopharm. 2001, 52, 203–210. [Google Scholar] [CrossRef]
- Herrador, M.A.; Asuero, A.G.; Gonzalez, A.G. Estimation of the uncertainty of indirect measurements from the propagation of distributions by using the Monte-Carlo method: An overview. Chemom. Intell. Lab. Syst. 2005, 79, 115–122. [Google Scholar] [CrossRef]
- Nompari, L.; Orlandini, S.; Pasquini, B.; Campa, C.; Rovini, M.; Bubba, M.D.; Furlanetto, S. Quality by design approach in the development of an ultra-high-performance liquid chromatography method for Bexsero meningococcal group B vaccine. Talanta 2018, 178, 552–562. [Google Scholar] [CrossRef]
- Ahmed, Z.Z.; Khan, F.N.; Shaikh, D.A. Reverse engineering and formulation by QBD of olopatadine hydrochloride ophthalmic solution. J. Pharm. Investig. 2018, 48, 279–293. [Google Scholar] [CrossRef]
- Debrus, B.; Guillarme, D.; Rudaz, S. Improved quality-by-design compliant methodology for method development in reversed-phase liquid chromatography. J. Pharm. Biomed. Anal. 2013, 84, 215–223. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, J.S.; You, Y.H.; Park, H.J.; Lee, S.; Park, J.S.; Woo, J.S.; Hwang, S.J. Development and optimization of a novel oral controlled delivery system for tamsulosin hydrochloride using response surface methodology. Int. J. Pharm. 2007, 341, 97–104. [Google Scholar] [CrossRef]
- Garala, K.C.; Patel, J.M.; Dhingani, A.P.; Dharamsi, A.T. Preparation and evaluation of agglomerated crystals by crystallo-co-agglomeration: An integrated approach of principal component analysis and Box-Behnken experimental design. Int. J. Pharm. 2013, 452, 135–156. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kang, Z.; Thapa, P.; Jin, Y.S.; Park, J.W.; Lim, H.J.; Lee, J.Y.; Lee, S.W.; Seo, M.H.; Kim, M.-S.; et al. Development of sorafenib loaded nanoparticles to improve oral bioavailability using a quality by design approach. Int. J. Pharm. 2019, 566, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Feng, X.; Williams, R.O., III; Zhang, F. Characterization of amorphous solid dispersions. J. Pharm. Investig. 2018, 48, 19–41. [Google Scholar] [CrossRef]
- Kumar, R.; Siril, P.F. Ultrafine carbamazepine nanoparticles with enhanced water solubility and rate of dissolution. RSC Adv. 2014, 4, 48101–48108. [Google Scholar] [CrossRef]
- Wu, W.; Wang, L.; Wang, L.; Zu, Y.; Wang, S.; Liu, P.; Zhao, X. Preparation of honokiol nanoparticles by liquid antisolvent precipitation technique, characterization, pharmacokinetics, and evaluation of inhibitory effect on HepG2 cells. Int. J. Nanomed. 2018, 13, 5469–5483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pu, Y.; Wen, X.; Li, Y.; Wang, D.; Foster, N.R.; Chen, J.F. Ultrafine clarithromycin nanoparticles via anti-solvent precipitation in subcritical water: Effect of operating parameters. Powder Technol. 2017, 305, 125–131. [Google Scholar] [CrossRef]












| QTTP Elements | Target | Justification |
|---|---|---|
| Dosage form | Nanosuspension | Small particles in the nanosuspension increase bioavailability by improving solubility and the dissolution rate [18]. |
| Route of administration | Oral | Oral administration is familiar to patients and results in high compliance with the medication. |
| Dosage strength | 100 mg (5 mg/mL or 10 mg/mL) | The usual dose of resveratrol is 100 mg. |
| Stability | Long-term stability for 2 years (at least 6 months) | This is the minimum period to confirm the stability of the final product. |
| Pharmacokinetics | Higher AUC0–12 h and Cmax, with lower Tmax | Improved bioavailability and therapeutic efficacy are achieved through increased drug absorption compared to suspension of resveratrol raw material. |
| CQA elements | Target | Justification |
| Particle size (z-average) | <250 nm | Commercial nano-formulation products have particle sizes <400 nm. To develop a product superior to a conventional commercial product, a particle size <250 nm is desired. |
| Particle size (d90 *) | <1 μm | All particle sizes of the nanosuspension should be <1 μm. |
| Zeta potential | >±20 mV | To disperse the particles, a repulsive force must be maintained on the particle surface. The desirable zeta potential value corresponding to electrostatic and steric stabilization interactions is >±20 mV [24]. |
| Drug content | 95.0–105.0% | The drug content has a high effect on safety and efficacy. |
| CQAs | Initial Risk Level | Updated Risk Level | ||||
|---|---|---|---|---|---|---|
| Particle Size | Zeta Potential | Drug Content | Particle Size | Zeta Potential | Drug Content | |
| Resveratrol concentration | High | Low | Low | Low | Low | Low |
| Stabilizer type | High | High | Low | Low | Low | Low |
| Stabilizer concentration | High | High | Low | High | High | Low |
| Solvent type | High | Low | Low | Low | Low | Low |
| Ratio of solvent/anti-solvent | High | Medium | Low | Low | Low | Low |
| Mixing speed | High | Low | Low | Medium | Low | Low |
| Mixing time | High | Low | Low | Low | Low | Low |
| Injection rate (solvent) | High | Low | Low | Medium | Low | Low |
| Temperature | High | Low | Low | Medium | Low | Low |
| Formulation Variables | Level Used | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| X1 = Concentration of PVP VA64 (mg/mL) | 5 | 7.5 | 10 |
| X2 = Concentration of PVP K12 (mg/mL) | 5 | 7.5 | 30 |
| X3 = Concentration of SLS (mg/mL) | 0.5 | 1 | 1.5 |
| Response variables | Goal | ||
| Y1 = particle size (z-average, initial) | <250 nm | ||
| Y2 = particle size (z-average, 1 day) | <250 nm | ||
| Y3 = particle size (z-average, 3 days) | <250 nm | ||
| Y4 = particle size (z-average, 7 days) | <250 nm | ||
| Y5 = particle size (d90, initial) | <1 µm | ||
| Y6 = particle size (d90, 1 day) | <1 µm | ||
| Y7 = particle size (d90, 3 days) | <1 µm | ||
| Y8 = particle size (d90, 7 days) | <1 µm | ||
| Y9 = zeta potential (ζ) | >±20 mV | ||
| StandardOrder | Parameters | Responses | Zeta Potential (mV) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | Particle Size (Z-Average, nm) | Particle Size (d90, nm) | ||||||||
| Initial | 1 Days | 3 Days | 7 Days | Initial | 1 Days | 3 Days | 7 Days | |||||
| Box–Behnken design | ||||||||||||
| 1 | 5.0 | 5.0 | 1.0 | 108.1 | 147.7 | 163.8 | 171.0 | 186.4 | 238.5 | 254.3 | 283.2 | −45.12 |
| 2 | 10.0 | 5.0 | 1.0 | 46.5 | 138.9 | 155.2 | 170.4 | 158.8 | 246.9 | 250.1 | 257.5 | −38.02 |
| 3 | 5.0 | 10.0 | 1.0 | 139.3 | 191.7 | 210.8 | 223.0 | 220.5 | 290.4 | 335.3 | 340.7 | −39.02 |
| 4 | 10.0 | 10.0 | 1.0 | 44.7 | 147.7 | 171.7 | 186.2 | 179.3 | 264.2 | 289.6 | 291.4 | −30.63 |
| 5 | 5.0 | 7.5 | 0.5 | 165.5 | 191.6 | 208.5 | 208.5 | 256.0 | 320.8 | 336.6 | 324.3 | −32.70 |
| 6 | 10.0 | 7.5 | 0.5 | 64.4 | 216.2 | 228.9 | 228.3 | 186.3 | 331.3 | 355.7 | 320.4 | −34.66 |
| 7 | 5.0 | 7.5 | 1.5 | 87.0 | 155.1 | 169.6 | 177.6 | 157.9 | 235.1 | 262.6 | 263.8 | −42.55 |
| 8 | 10.0 | 7.5 | 1.5 | 33.7 | 101.7 | 130.7 | 145.2 | 184.0 | 237.2 | 262.2 | 258.0 | −35.57 |
| 9 | 7.5 | 5.0 | 0.5 | 127.0 | 217.1 | 230.8 | 233.8 | 225.5 | 328.8 | 333.8 | 339.5 | −41.36 |
| 10 | 7.5 | 10.0 | 0.5 | 155.5 | 257.7 | 274.8 | 270.8 | 269.0 | 373.0 | 426.8 | 408.0 | −32.44 |
| 11 | 7.5 | 5.0 | 1.5 | 68.7 | 153.4 | 168.5 | 175.1 | 174.0 | 247.7 | 263.9 | 266.5 | −41.25 |
| 12 | 7.5 | 10.0 | 1.5 | 69.9 | 166.1 | 184.3 | 198.1 | 185.3 | 274.3 | 297.6 | 302.8 | −35.52 |
| 13 | 7.5 | 7.5 | 1.0 | 85.6 | 203.2 | 211.8 | 220.4 | 200.6 | 309.5 | 344.7 | 325.6 | −38.02 |
| 14 | 7.5 | 7.5 | 1.0 | 84.0 | 203.5 | 217.4 | 217.5 | 189.1 | 301.3 | 327.8 | 336.5 | −42.06 |
| 15 | 7.5 | 7.5 | 1.0 | 77.5 | 200.3 | 217.1 | 215.7 | 194.8 | 306.1 | 326.3 | 335.9 | −38.67 |
| 16 | 7.5 | 7.5 | 1.0 | 82.8 | 204.2 | 220.1 | 221.9 | 198.6 | 306.9 | 331.5 | 322.2 | −39.84 |
| 17 | 7.5 | 7.5 | 1.0 | 76.7 | 199.3 | 215.0 | 216.6 | 193.7 | 302.3 | 336.1 | 317.9 | −37.57 |
| Full factorial design | ||||||||||||
| 1 | 0.5 | 20 | 500 | 48.0 | 133.0 | 151.9 | 157.8 | 170.1 | 245.3 | 254.2 | 260.3 | −32.97 |
| 2 | 1.5 | 20 | 500 | 53.3 | 136.6 | 158.6 | 163.9 | 188.0 | 241.7 | 265.9 | 264.3 | −36.24 |
| 3 | 0.5 | 30 | 500 | 49.6 | 136.1 | 157.3 | 164.1 | 184.7 | 247.7 | 269.4 | 265.7 | −38.07 |
| 4 | 1.5 | 30 | 500 | 52.7 | 132.8 | 152.7 | 160.4 | 183.6 | 241.0 | 261.7 | 271.9 | −39.07 |
| 5 | 0.5 | 20 | 1000 | 52.4 | 136.9 | 156.0 | 162.7 | 168.2 | 232.5 | 262.0 | 265.1 | −38.02 |
| 6 | 1.5 | 20 | 1000 | 54.7 | 140.4 | 157.6 | 163.5 | 192.2 | 245.5 | 253.8 | 261.5 | −39.60 |
| 7 | 0.5 | 30 | 1000 | 46.0 | 132.2 | 156.1 | 162.0 | 161.7 | 236.4 | 255.5 | 274.4 | −34.59 |
| 8 | 1.5 | 30 | 1000 | 43.1 | 127.6 | 152.2 | 160.2 | 175.2 | 245.6 | 243.8 | 277.1 | −35.91 |
| 9 | 1.0 | 25 | 750 | 49.9 | 135.2 | 158.2 | 165.7 | 159.2 | 241.1 | 256.3 | 262.3 | −36.19 |
| Response | R2 | Pred. R2 | PRESS | %CV | p-Value | Lack of Fit |
|---|---|---|---|---|---|---|
| Y1 | 0.9907 | 0.8915 | 2515.8 | 6.21 | <0.0001 | 0.1491 |
| Y2 | 0.9959 | 0.9465 | 1220.5 | 2.01 | <0.0001 | 0.0724 |
| Y3 | 0.9911 | 0.8850 | 2300.2 | 2.54 | <0.0001 | 0.0783 |
| Y4 | 0.9858 | 0.9334 | 995.7 | 2.25 | <0.0001 | 0.0869 |
| Y5 | 0.9753 | 0.7569 | 3522.4 | 3.38 | <0.0001 | 0.1300 |
| Y6 | 0.9929 | 0.9483 | 1304 | 1.64 | <0.0001 | 0.1638 |
| Y7 | 0.9726 | 0.6543 | 11840.4 | 3.69 | <0.0001 | 0.0948 |
| Y8 | 0.9503 | 0.6010 | 9548.2 | 3.91 | 0.0002 | 0.1357 |
| Y9 | 0.8629 | 0.0684 | 234.1 | 5.47 | 0.0087 | 0.3160 |
| Regression equation of the fitted model | ||||||
| Y1 = 81.33 − 38.83X1 + 7.38X2 − 31.65X3 − 8.27X1X2 + 11.96X1X3 − 6.82X2X3 − 7.16X12 + 10.48X22 + 13.47X32. | ||||||
| Y2 = 202.07 − 10.20X1 + 13.27X2 − 38.28X3 − 8.80X1X2 − 19.50X1X3 − 6.99X2X3 − 39.01X12 − 6.57X22 + 3.07X32. | ||||||
| Y3 = 216.29 − 8.29X1 + 15.41X2 − 36.24X3 − 7.63X1X2 − 14.83X1X3 − 7.05X2X3 − 35.54X12 − 5.38X22 + 3.69X32. | ||||||
| Y4 = 218.89 − 6.25X1 + 16.00X2 − 30.68X3 − 9.04X1X2 − 13.07X1X3 − 30.11X12. | ||||||
| Y5 = 195.36 − 14.06X1 + 13.68X2 − 29.47X3 + 23.94X1X3 − 8.06X2X3 − 13.26X12 + 4.14X22 + 13.93X32. | ||||||
| Y6 = 305.23 − 0.64X1 + 17.50X2 − 44.93X3 − 8.66X1X2 − 4.38X2X3 − 35.03X12 − 10.20X22 + 10.90X32. | ||||||
| Y7 = 333.28 − 3.89X1 + 30.90X2 − 45.82X3 − 10.38X1X2 − 4.90X1X3 − 38.61X12 − 12.35X22 + 9.61X32. | ||||||
| Y8 = 327.93 − 10.70X1 + 24.51X2 − 37.76X3 − 5.92X1X2 + 1.24X1X3 − 8.03X2X3 − 35.11X12. | ||||||
| Y9 = -39.28 + 2.56X1 + 3.52X2 − 1.72X3 + 0.32X1X2 + 2.23X1X3 − 0.80X2X3 + 1.15X12 + 1.77X32. | ||||||
| Formulations | AUC0–12 h (ng∙h/mL) | Cmax (ng/mL) | Tmax (h) |
|---|---|---|---|
| Resveratrol raw material | 240.3 ± 42.2 | 52.5 ± 11.7 | 1.06 ± 0.83 |
| Resveratrol nanosuspension | 387.0 ± 26.0 a | 301.4 ± 79.6 a | 0.44 ± 0.13 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuk, D.-H.; Ha, E.-S.; Ha, D.-H.; Sim, W.-Y.; Lee, S.-K.; Jeong, J.-S.; Kim, J.-S.; Baek, I.-h.; Park, H.; Choi, D.H.; et al. Development of a Resveratrol Nanosuspension Using the Antisolvent Precipitation Method without Solvent Removal, Based on a Quality by Design (QbD) Approach. Pharmaceutics 2019, 11, 688. https://doi.org/10.3390/pharmaceutics11120688
Kuk D-H, Ha E-S, Ha D-H, Sim W-Y, Lee S-K, Jeong J-S, Kim J-S, Baek I-h, Park H, Choi DH, et al. Development of a Resveratrol Nanosuspension Using the Antisolvent Precipitation Method without Solvent Removal, Based on a Quality by Design (QbD) Approach. Pharmaceutics. 2019; 11(12):688. https://doi.org/10.3390/pharmaceutics11120688
Chicago/Turabian StyleKuk, Do-Hoon, Eun-Sol Ha, Dong-Hyun Ha, Woo-Yong Sim, Seon-Kwang Lee, Ji-Su Jeong, Jeong-Soo Kim, In-hwan Baek, Heejun Park, Du Hyung Choi, and et al. 2019. "Development of a Resveratrol Nanosuspension Using the Antisolvent Precipitation Method without Solvent Removal, Based on a Quality by Design (QbD) Approach" Pharmaceutics 11, no. 12: 688. https://doi.org/10.3390/pharmaceutics11120688