Bi-Layered Polymer Carriers with Surface Modification by Electrospinning for Potential Wound Care Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Carriers
2.2.1. Solvent Casting
2.2.2. Electrospinning
2.2.3. 3D Printing
2.2.4. Crosslinking
2.3. Characterization Methods
2.3.1. Visualization
2.3.2. Texture Analysis
2.3.3. Solid-State Characterization
2.3.4. Stability Study
2.4. Behavior of Bi-Layered Carriers in Biorelevant Conditions and During DDSs Preparation
2.4.1. Swelling and Degradation in Aqueous Environment
2.4.2. Simulated Bioadhesion Study
2.4.3. Safety of Bi-Layered Carriers and the Effect of Surface Modification on Cell Viability
2.4.4. Surface Behavior During Inkjet Printing
2.5. Data Analysis
3. Results and discussion
3.1. Characterization of Solvent Casted (SC) Films, Electrospun Nanofibers (NFs) and 3D Printed Mats
3.2. Preparation and Structure of Bi-Layered Carriers
3.3. Physical Properties
3.4. Stability and Solid-State Characterization
3.5. Bioadhesion within Simulated Wound Fluid
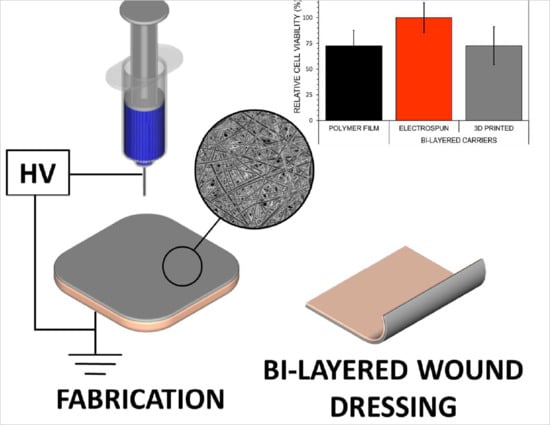
3.6. Safety of Bi-Layered Carriers and the Effect of Surface Modification on Cell Viability
3.7. Surface Properties During Inkjet Printing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.E.; Eccleston, G.M. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef]
- Percival, S.L.; Hill, K.E.; Williams, D.W.; Hooper, S.J.; Thomas, D.W.; Costerton, J.W. A review of the scientific evidence for biofilms in wounds. Wound Repair Regen. 2012, 20, 647–657. [Google Scholar] [CrossRef]
- Rambhia, K.J.; Ma, P.X. Controlled drug release for tissue engineering. J. Control. Release 2015, 219, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Zahedi, P.; Rezaeian, I.; Ranaei-Siadat, S.-O.; Jafari, S.-H.; Supaphol, P. A review on wound dressings with an emphasis on electrospun nanofibrous polymeric bandages. Polym. Adv. Technol. 2010, 21, 77–95. [Google Scholar] [CrossRef]
- Dwivedi, C.; Pandey, H.; Pandey, A.C.; Patil, S.; Ramteke, P.W.; Laux, P.; Luch, A.; Singh, A.V. In vivo biocompatibility of electrospun biodegradable dual carrier (antibiotic + growth factor) in a mouse model—Implications for rapid wound healing. Pharmaceutics 2019, 11, 180. [Google Scholar] [CrossRef] [Green Version]
- Buanz, A.B.M.; Belaunde, C.C.; Soutari, N.; Tuleu, C.; Gul, O.; Gaisford, S. Ink-jet printing versus solvent casting to prepare oral films: Effect on mechanical properties and physical stability. Int. J. Pharm. 2015, 494, 611–618. [Google Scholar] [CrossRef]
- Preis, M.; Knop, K.; Breitkreutz, J. Mechanical strength test for orodispersible and buccal films. Int. J. Pharm. 2014, 461, 22–29. [Google Scholar] [CrossRef]
- Rodríguez-Tobías, H.; Morales, G.; Grande, D. Comprehensive review on electrospinning techniques as versatile approaches toward antimicrobial biopolymeric composite fibers. Mater. Sci. Eng. C 2019, 101, 306–322. [Google Scholar] [CrossRef]
- Miguel, S.P.; Figueira, D.R.; Simões, D.; Ribeiro, M.P.; Coutinho, P.; Ferreira, P.; Correia, I.J. Electrospun polymeric nanofibres as wound dressings: A review. Colloids Surf. B Biointerfaces 2018, 169, 60–71. [Google Scholar] [CrossRef]
- Agarwal, S.; Wendorff, J.H.; Greiner, A. Use of electrospinning technique for biomedical applications. Polymer 2008, 49, 5603–5621. [Google Scholar] [CrossRef] [Green Version]
- Palo, M.; Özliseli, E.; Sen Karaman, D.; Kogermann, K. Electrospun biocomposite fibers for wound healing applications. In Green Electrospinning; Horzum, N., Demir, M.M., Muñoz-Espí, R., Crespy, D., Eds.; DeGruyter: Berlin, Germany; Boston, MA, USA, 2019; pp. 265–320. [Google Scholar]
- Hölzl, K.; Lin, S.; Tytgat, L.; Van Vlierberghe, S.; Gu, L.; Ovsianikov, A. Bioink properties before, during and after 3D bioprinting. Biofabrication 2016, 8, 032002. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D Bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Sandler, N.; Preis, M. Printed Drug-Delivery Systems for Improved Patient Treatment. Trends Pharmacol. Sci. 2016, 37, 1070–1080. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. Medical applications for 3D Printing: Current and projected uses. Pharm. Ther. 2014, 39, 704–711. [Google Scholar]
- Tort, S.; Acartürk, F.; Beşikci, A. Evaluation of three-layered doxycycline-collagen loaded nanofiber wound dressing. Int. J. Pharm. 2017, 529, 642–653. [Google Scholar] [CrossRef]
- Tan, L.; Hu, J.; Zhao, H. Design of bilayered nanofibrous mats for wound dressing using an electrospinning technique. Mater. Lett. 2015, 156, 46–49. [Google Scholar] [CrossRef]
- Alhusein, N.; de Bank, P.A.; Blagbrough, I.S.; Bolhuis, A. Killing bacteria within biofilms by sustained release of tetracycline from triple-layered electrospun micro/nanofibre matrices of polycaprolactone and poly(ethylene-co-vinyl acetate). Drug Deliv. Transl. Res. 2013, 3, 531–541. [Google Scholar] [CrossRef] [Green Version]
- Thabet, Y.; Lunter, D.; Breitkreutz, J. Continuous manufacturing and analytical characterization of fixed-dose, multilayer orodispersible films. Eur. J. Pharm. Sci. 2018, 117, 236–244. [Google Scholar] [CrossRef]
- Maurmann, N.; Pereira, D.P.; Burguez, D.; de S Pereira, F.D.A.; Inforcatti Neto, P.; Rezende, R.A.; Gamba, D.; da Silva, J.V.L.; Pranke, P. Mesenchymal stem cells cultivated on scaffolds formed by 3D printed PCL matrices, coated with PLGA electrospun nanofibers for use in tissue engineering. Biomed. Phys. Eng. Express 2017, 3, 1–15. [Google Scholar] [CrossRef]
- Maver, T.; Smrke, D.M.; Kurečič, M.; Gradišnik, L.; Maver, U.; Kleinschek, K.S. Combining 3D printing and electrospinning for preparation of pain-relieving wound-dressing materials. J. Sol-Gel Sci. Technol. 2018, 88, 33–48. [Google Scholar] [CrossRef]
- Scoutaris, N.; Ross, S.; Douroumis, D. Current trends on medical and pharmaceutical applications of inkjet printing technology. Pharm. Res. 2016, 33, 1799–1816. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Ma, X.; Gou, M.; Mei, D.; Zhang, K.; Chen, S. 3D printing of functional biomaterials for tissue engineering. Curr. Opin. Biotechnol. 2016, 40, 103–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daly, R.; Harrington, T.S.; Martin, G.D.; Hutchings, I.M. Inkjet printing for pharmaceutics—A review of research and manufacturing. Int. J. Pharm. 2015, 494, 554–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Setti, L.; Fraleoni-Morgera, A.; Ballarin, B.; Filippini, A.; Frascaro, D.; Piana, C. An amperometric glucose biosensor prototype fabricated by thermal inkjet printing. Biosens. Bioelectron. 2005, 20, 2019–2026. [Google Scholar] [CrossRef]
- Derby, B. Additive manufacture of ceramics components by inkjet printing. Engineering 2015, 1, 113–123. [Google Scholar] [CrossRef] [Green Version]
- Määttänen, A.; Ihalainen, P.; Pulkkinen, P.; Wang, S.; Tenhu, H.; Peltonen, J. Inkjet-printed gold electrodes on paper: Characterization and functionalization. ACS Appl. Mater. Interfaces 2012, 4, 955–964. [Google Scholar] [CrossRef]
- Derby, B. Inkjet printing of functional and structural materials: Fluid property requirements, feature stability, and resolution. Annu. Rev. Mater. Res. 2010, 40, 395–414. [Google Scholar] [CrossRef]
- Montenegro-Nicolini, M.; Reyes, P.E.; Jara, M.O.; Vuddanda, P.R.; Neira-Carrillo, A.; Butto, N.; Velaga, S.; Morales, J.O. The effect of inkjet printing over polymeric films as potential buccal biologics delivery systems. AAPS PharmSciTech 2018, 19, 3376–3387. [Google Scholar] [CrossRef]
- Genina, N.; Fors, D.; Palo, M.; Peltonen, J.; Sandler, N. Behavior of printable formulations of loperamide and caffeine on different substrates—Effect of print density in inkjet printing. Int. J. Pharm. 2013, 453, 488–497. [Google Scholar] [CrossRef]
- Genina, N.; Fors, D.; Vakili, H.; Ihalainen, P.; Pohjala, L.; Ehlers, H.; Kassamakov, I.; Haeggström, E.; Vuorela, P.; Peltonen, J.; et al. Tailoring Controlled-Release Oral Dosage Forms by Combining Inkjet and Flexographic Printing Techniques. Eur. J. Pharm. Sci. 2012, 47, 615–623. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Chen, X.; Mohy Eldin, M.S.; Kenawy, E.R.S. Crosslinked poly(vinyl alcohol) hydrogels for wound dressing applications: A review of remarkably blended polymers. Arab. J. Chem. 2015, 8, 1–14. [Google Scholar] [CrossRef] [Green Version]
- DeMerlis, C.C.; Schoneker, D.R. Review of the oral toxicity of polyvinyl alcohol (PVA). Food Chem. Toxicol. 2003, 41, 319–326. [Google Scholar] [CrossRef]
- Abdelgawad, A.M.; Hudson, S.M.; Rojas, O.J. Antimicrobial wound dressing nanofiber mats from multicomponent (chitosan/silver-NPs/polyvinyl alcohol) systems. Carbohydr. Polym. 2014, 100, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Charernsriwilaiwat, N.; Rojanarata, T.; Ngawhirunpat, T.; Opanasopit, P. Electrospun chitosan/polyvinyl alcohol nanofibre mats for wound healing. Int. Wound J. 2014, 11, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Bolto, B.; Tran, T.; Hoang, M.; Xie, Z. Crosslinked poly(vinyl alcohol) membranes. Prog. Polym. Sci. 2009, 34, 969–981. [Google Scholar] [CrossRef]
- Shalumon, K.T.; Anulekha, K.H.; Nair, S.V.; Nair, S.V.; Chennazhi, K.P.; Jayakumar, R. Sodium alginate/poly(vinyl alcohol)/nano ZnO composite nanofibers for antibacterial wound dressings. Int. J. Biol. Macromol. 2011, 49, 247–254. [Google Scholar] [CrossRef]
- Sobhanian, P.; Khorram, M.; Hashemi, S.S.; Mohammadi, A. Development of nanofibrous collagen-grafted poly (vinyl alcohol)/gelatin/alginate scaffolds as potential skin substitute. Int. J. Biol. Macromol. 2019, 130, 977–987. [Google Scholar] [CrossRef]
- Kim, J.O.; Park, J.K.; Kim, J.H.; Jin, S.G.; Yong, C.S.; Li, D.X.; Choi, J.Y.; Woo, J.S.; Yoo, B.K.; Lyoo, W.S.; et al. Development of polyvinyl alcohol-sodium alginate gel-matrix-based wound dressing system containing nitrofurazone. Int. J. Pharm. 2008, 359, 79–86. [Google Scholar] [CrossRef]
- European Medicines Agency. Guideline on Stability Testing: Stability Testing of Existing Active Substances and Related Finished Products; European Medicines Agency: London, UK, 2003; pp. 1–18. [Google Scholar]
- Miraftab, M.; Saifullah, A.N.; Çay, A. Physical stabilisation of electrospun poly(vinyl alcohol) nanofibres: Comparative study on methanol and heat-based crosslinking. J. Mater. Sci. 2015, 50, 1943–1957. [Google Scholar] [CrossRef]
- Tamm, I.; Heinämäki, J.; Laidmäe, I.; Rammo, L.; Paaver, U.; Ingebrigtsen, S.G.; Škalko-Basnet, N.; Halenius, A.; Yliruusi, J.; Pitkänen, P.; et al. Development of suberin fatty acids and chloramphenicol-loaded antimicrobial electrospun nanofibrous mats intended for wound therapy. J. Pharm. Sci. 2016, 105, 1239–1247. [Google Scholar] [CrossRef]
- Huang, K.-T.; Fang, Y.-L.; Hsieh, P.-S.; Li, C.-C.; Dai, N.-T.; Huang, C.-J. Zwitterionic nanocomposite hydrogels as effective wound dressings. J. Mater. Chem. B 2016, 4, 4206–4215. [Google Scholar] [CrossRef]
- Sjöholm, E.; Sandler, N. Additive manufacturing of personalized orodispersible warfarin films. Int. J. Pharm. 2019, 564, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Birck, C.; Degoutin, S.; Tabary, N.; Miri, V.; Bacquet, M. New crosslinked cast films based on poly(vinyl alcohol): Preparation and physico-chemical properties. Express Polym. Lett. 2014, 8, 941–952. [Google Scholar] [CrossRef] [Green Version]
- Fuchs, S.; Hartmann, J.; Mazur, P.; Reschke, V.; Siemens, H.; Wehlage, D.; Ehrmann, A. Electrospinning of biopolymers and biopolymer blends. J. Chem. Pharm. Sci. 2017, 974, 2115. [Google Scholar]
- Hansen, E.F.; Derrick, M.R.; Schilling, M.R.; Garcia, R. The effects of solution application on some mechanical and physical properties of thermoplastic amorphous polymers used in conservation: Poly(vinyl acetate)s. J. Am. Inst. Conserv. 1991, 30, 203–213. [Google Scholar] [CrossRef]
- Stone, S.A.; Gosavi, P.; Athauda, T.J.; Ozer, R.R. In situ citric acid crosslinking of alginate/polyvinyl alcohol electrospun nanofibers. Mater. Lett. 2013, 112, 32–35. [Google Scholar] [CrossRef]
- Morgado, P.I.; Lisboa, P.F.; Ribeiro, M.P.; Miguel, S.P.; Simões, P.C.; Correia, I.J.; Aguiar-Ricardo, A. Poly(vinyl alcohol)/chitosan asymmetrical membranes: Highly controlled morphology toward the ideal wound dressing. J. Memb. Sci. 2014, 469, 262–271. [Google Scholar] [CrossRef]
- Qin, Y. Absorption characteristics of alginate wound dressings. J. Appl. Polym. Sci. 2004, 91, 953–957. [Google Scholar] [CrossRef]
- El-Din, H.M.N.; Alla, S.G.A.; El-Naggar, A.W.M. Swelling, thermal and mechanical properties of poly(vinyl alcohol)/sodium alginate hydrogels synthesized by electron beam irradiation. J. Macromol. Sci. Part A Pure Appl. Chem. 2007, 44, 291–297. [Google Scholar] [CrossRef]
- Safi, S.; Morshed, M.; Hosseini Ravandi, S.A.; Ghiaci, M. Study of electrospinning of sodium alginate, blended solutions of sodium alginate/poly(vinyl alcohol) and sodium alginate/poly(ethylene oxide). J. Appl. Polym. Sci. 2007, 104, 3245–3255. [Google Scholar] [CrossRef]
- Lawrie, G.; Keen, I.; Drew, B.; Chandler-Temple, A.; Rintoul, L.; Fredericks, P.; Grøndahl, L. Interactions between alginate and chitosan biopolymers characterized using FTIR and XPS. Biomacromolecules 2007, 8, 2533–2541. [Google Scholar] [CrossRef] [PubMed]
- Mansur, H.S.; Sadahira, C.M.; Souza, A.N.; Mansur, A.A.P. FTIR spectroscopy characterization of poly (vinyl alcohol) hydrogel with different hydrolysis degree and chemically crosslinked with glutaraldehyde. Mater. Sci. Eng. C 2008, 28, 539–548. [Google Scholar] [CrossRef]
- Mallapragada, S.K.; Peppas, N.A. Dissolution mechanism of semicrystalline poly(vinyl alcohol) in water. J. Polym. Sci. Part B Polym. Phys. 1996, 34, 1339–1346. [Google Scholar] [CrossRef]
- Gohil, J.M.; Bhattacharya, A.; Ray, P. Studies on the cross-linking of poly (vinyl alcohol). J. Polym. Res. 2006, 13, 161–169. [Google Scholar] [CrossRef]
- El-Sayed, S.; Mahmoud, K.H.; Fatah, A.A.; Hassen, A. DSC, TGA and dielectric properties of carboxymethyl cellulose/polyvinyl alcohol blends. Phys. B Condens. Matter 2011, 406, 4068–4076. [Google Scholar] [CrossRef]
- Soares, J.P.; Santos, J.E.; Chierice, G.O.; Cavalheiro, E.T.G. Thermal behavior of alginic acid and its sodium salt. Eclet. Quim. 2004, 29, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, F.C.; Calixto, G.; Hatakeyama, I.N.; Luz, G.M.; Gremião, M.P.D.; Chorilli, M. Rheological, mechanical, and bioadhesive behavior of hydrogels to optimize skin delivery systems. Drug Dev. Ind. Pharm. 2013, 39, 1750–1757. [Google Scholar] [CrossRef]
- Singh, S.; Jain, S.; Muthu, M.S.; Tiwari, S.; Tilak, R. Preparation and evaluation of buccal bioadhesive films containing clotrimazole. AAPS PharmSciTech 2008, 9, 660–667. [Google Scholar] [CrossRef]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound dressings—A review. BioMedicine 2015, 5, 24–28. [Google Scholar] [CrossRef]
- Rippon, M.; White, R.; Davies, P. Skin adhesives and their role in wound dressings. Wounds UK 2007, 3, 76–86. [Google Scholar]
- Sood, A.; Granick, M.S.; Tomaselli, N.L. Wound dressings and comparative effectiveness data. Adv. Wound Care 2014, 3, 511–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horstmann, M.; Müller, W.; Asmussen, B. Principles of skin adhesion and methods for measuring adhesion of transdermal systems. In Bioadhesive Drug Delivery Systems: Fundamentals, Novel Approaches, and Development; Mathiowitz, E., Chickering, D.E., III, Lehr, C.-M., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 1999; pp. 175–196. [Google Scholar]
- Matos-Pérez, C.R.; White, J.D.; Wilker, J.J. Polymer composition and substrate influences on the adhesive bonding of a biomimetic, cross-linking polymer. J. Am. Chem. Soc. 2012, 134, 9498–9505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Substances Generally Recognized as Safe, 21 C.F.R. § 182U.S.; Food and Drug Administration; FDA: Silver Spring, MD, USA, 2019.
- Orive, G.; Ponce, S.; Hernández, R.M.; Gascón, A.R.; Igartua, M.; Pedraz, J.L. Biocompatibility of microcapsules for cell immobilization elaborated with different type of alginates. Biomaterials 2002, 23, 3825–3831. [Google Scholar] [CrossRef]
- Alexandre, N.; Ribeiro, J.; Gärtner, A.; Pereira, T.; Amorim, I.; Fragoso, J.; Lopes, A.; Fernandes, J.; Costa, E.; Santos-Silva, A.; et al. Biocompatibility and hemocompatibility of polyvinyl alcohol hydrogel used for vascular grafting—In vitro and in vivo studies. J. Biomed. Mater. Res. Part A 2014, 102, 4262–4275. [Google Scholar]
- Ruprecht, V.; Monzo, P.; Ravasio, A.; Yue, Z.; Makhija, E.; Strale, P.O.; Gauthier, N.; Shivashankar, G.V.; Studer, V.; Albiges-Rizo, C.; et al. How cells respond to environmental cues—Insights from bio-functionalized substrates. J. Cell Sci. 2017, 130, 51–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, N.D.; Gentleman, E. The role of material structure and mechanical properties in cell–matrix interactions. J. Mater. Chem. B 2014, 2, 2345–2356. [Google Scholar] [CrossRef] [Green Version]
- Richbourg, N.R.; Peppas, N.A.; Sikavitsas, V.I. Tuning the biomimetic behavior of scaffolds for regenerative medicine through surface modifications. J. Tissue Eng. Regen. Med. 2019, 13, 1275–1293. [Google Scholar] [CrossRef]
- Amani, H.; Arzaghi, H.; Bayandori, M.; Dezfuli, A.S.; Pazoki-Toroudi, H.; Shafiee, A.; Moradi, L. Controlling cell behavior through the design of biomaterial surfaces: A focus on surface modification techniques. Adv. Mater. Interfaces 2019, 6, 1900572. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Bružauskaitė, I.; Bironaitė, D.; Bagdonas, E.; Bernotienė, E. Scaffolds and cells for tissue regeneration: Different scaffold pore sizes—Different cell effects. Cytotechnology 2016, 68, 355–369. [Google Scholar] [CrossRef] [Green Version]
- Palo, M.; Kogermann, K.; Laidmäe, I.; Meos, A.; Preis, M.; Heinämäki, J.; Sandler, N. Development of oromucosal dosage forms by combining electrospinning and inkjet printing. Mol. Pharm. 2017, 14, 808–820. [Google Scholar] [CrossRef] [PubMed]
- Palo, M.; Öhman, J.; Oja, T.; Sandler, N. Development of wound dressings for biofilm inhibition by means of inkjet printing. In Proceedings of the NIP32: International Conference on Digital Printing Technologies, Manchester, UK, 12–16 September 2016; pp. 1–3. [Google Scholar]










| Sample Treatment | Thickness a (mm) | Puncture Test a | Swelling Degree b (%) After 24 h | Degradation Degree b (%) After 7 h | |
|---|---|---|---|---|---|
| Burst Strength (N) | Distance at Break (mm) | ||||
| Solvent Cast (SC) Film | |||||
| non-crosslinked | 0.04 ± 0.01 | 40.2 ± 14.9 | 5.0 ± 2.1 | NA | NA |
| crosslinked | 0.03 ± 0.02 | 37.4 ± 30.2 | 3.1 ± 0.9 | ↑ 69 ± 19 | ↓ 1.2 ± 1.2 |
| Electrospun Nanofiber (NF) Mat | |||||
| non-crosslinked | NA | NA | NA | NA | NA |
| crosslinked | NA | NA | NA | ↑ 401 ± 52 | ↓ 4.9 ± 5.9 c |
| Patterned 3D Printed (3D) Mat | |||||
| non-crosslinked | 0.04 ± 0.01 | 5.3 ± 1.1 | 2.9 ± 0.7 | NA | NA |
| crosslinked | 0.05 ± 0.01 | 5.3 ± 1.3 | 2.6 ± 0.4 | ↑ 75 ± 39 | ↓ 6.7 ± 0.7 |
| Bi-Layered Solvent Cast/Nanofiber (SC/NF) Carrier | |||||
| non-crosslinked | 0.05 ± 0.01 | 36.8 ± 5.7 | 6.4 ± 0.8 | NA | NA |
| crosslinked | 0.09 ± 0.02 | 45.5 ± 12.4 | 3.4 ± 0.8 | ↑ 338 ± 35 | ↓ 0.2 ± 0.3 |
| Bi-Layered Solvent Cast /3D Printed (SC/3D) Carrier | |||||
| non-crosslinked | 0.08 ± 0.03 | 38.4 ± 31.2 | 4.3 ± 1.8 | NA | NA |
| crosslinked | 0.11 ± 0.04 | 31.3 ± 10.9 | 2.4 ± 0.4 | ↑ 133 ± 20 | ↓ 2.8 ± 0.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palo, M.; Rönkönharju, S.; Tiirik, K.; Viidik, L.; Sandler, N.; Kogermann, K. Bi-Layered Polymer Carriers with Surface Modification by Electrospinning for Potential Wound Care Applications. Pharmaceutics 2019, 11, 678. https://doi.org/10.3390/pharmaceutics11120678
Palo M, Rönkönharju S, Tiirik K, Viidik L, Sandler N, Kogermann K. Bi-Layered Polymer Carriers with Surface Modification by Electrospinning for Potential Wound Care Applications. Pharmaceutics. 2019; 11(12):678. https://doi.org/10.3390/pharmaceutics11120678
Chicago/Turabian StylePalo, Mirja, Sophie Rönkönharju, Kairi Tiirik, Laura Viidik, Niklas Sandler, and Karin Kogermann. 2019. "Bi-Layered Polymer Carriers with Surface Modification by Electrospinning for Potential Wound Care Applications" Pharmaceutics 11, no. 12: 678. https://doi.org/10.3390/pharmaceutics11120678