Sugar and Polymer Excipients Enhance Uptake and Splice-Switching Activity of Peptide-Dendrimer/Lipid/Oligonucleotide Formulations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Line and Culture Conditions
2.3. ON Transfection of Reporter Cell Lines Under Serum Conditions
2.4. Fluorescence Microscopy
2.5. Flow Cytometric Analysis
2.6. Luciferase Assay
2.7. RNA Expression Analysis of Splice-Correction
2.8. Effect of Lyophilization on Activity
2.9. Particle Size and Zeta Potential Measurements
2.10. Cell Viability
2.11. Animal Biodistribution Experiment
2.12. Data Analysis
3. Results and Discussion
3.1. Sugar Excipients Enhance Transfection Efficiency of G2-RR PDLO-Complexes in HeLa Luc/705 Reporter Cell Line
3.2. G2-RR PDLO-Complexes Display Transfection Efficiencies Superior to Lipofectamine 2000 (L2000) in the Presence of Polymer Excipients
3.3. Splice-Corrected mRNA Levels Confirmed the Enhancing Activity of Excipients on Transfection
3.4. PVA Excipients Are More Lyoprotective to the Formulations Compared to the PVP Excipients
3.5. Excipients Tested Are Non-Toxic to HeLa Luc/705 Cells
3.6. Selected Excipients Enhance Cellular Uptake of the Complexes and Luciferase Correction at Early Time-Points
3.7. Lead Excipients Enhance Efficacy of G2-RR PDLO-Complexes to Comparable Levels to L2000 in Different Reporter Cell Lines
3.8. Excipients Result in Smaller Sizes and Narrow Size Distribution of the Formulated Complexes
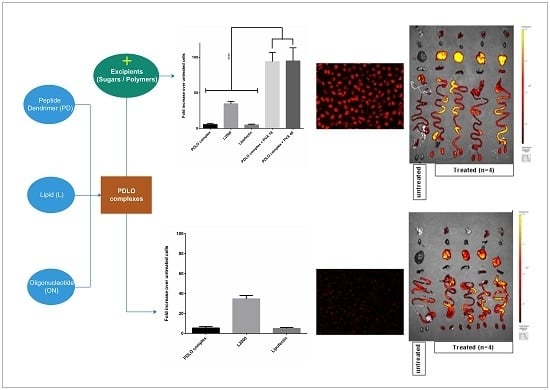
3.9. Lead Excipients Affect ON Biodistribution Profile in Mice
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Denkewalter, R.G.; Kolc, J.; Lukasavage, W.J. Macromolecular highly branched homogeneous compound based on lysine units 1981. U.S. Patent 4289872; Chemical Ab, 102, 79324, 4 September 1981. [Google Scholar]
- Aharoni, S.M.; Crosby, C.R.; Walsh, E.K. Size and solution properties of globular tert-butyloxycarbonyl-poly(α,iε-L-lysine). Macromolecules 1982, 15, 1093–1098. [Google Scholar] [CrossRef]
- Manikkath, J.; Hegde, A.R.; Parekh, H.S.; Mutalik, S. Peptide Dendrimers in Delivery of Bioactive Molecules to Skin. In Nanoscience in Dermatology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 89–97. ISBN 9780128029268. [Google Scholar]
- Chen, Q.-R. Branched co-polymers of histidine and lysine are efficient carriers of plasmids. Nucleic Acids Res. 2001, 29, 1334–1340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leng, Q.; Scaria, P.; Ioffe, O.B.; Woodle, M.; Mixson, A.J. A branched histidine/lysine peptide, H2K4b, in complex with plasmids encoding antitumor proteins inhibits tumor xenografts. J. Gene Med. 2006, 8, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Sugiyama, A.; Niidome, T.; Aoyagi, H. Characters of dendritic poly(l-lysine) analogues with the terminal lysines replaced with arginines and histidines as gene carriers in vitro. Biomaterials 2004, 25, 537–544. [Google Scholar] [CrossRef]
- Cai, X.; Jin, R.; Wang, J.; Yue, D.; Jiang, Q.; Wu, Y.; Gu, Z. Bioreducible Fluorinated Peptide Dendrimers Capable of Circumventing Various Physiological Barriers for Highly Efficient and Safe Gene Delivery. ACS Appl. Mater. Interfaces 2016, 8, 5821–5832. [Google Scholar] [CrossRef]
- Lampela, P.; Elomaa, M.; Ruponen, M.; Urtti, A.; Männistö, P.T.; Raasmaja, A. Different synergistic roles of small polyethylenimine and Dosper in gene delivery. J. Control. Release 2003, 88, 173–183. [Google Scholar] [CrossRef]
- Lampela, P.; Soininen, P.; Urtti, A.; Männistö, P.T.; Raasmaja, A. Synergism in gene delivery by small PEIs and three different nonviral vectors. Int. J. Pharm. 2004, 270, 175–184. [Google Scholar] [CrossRef]
- Welser, K.; Campbell, F.; Kudsiova, L.; Mohammadi, A.; Dawson, N.; Hart, S.L.; Barlow, D.J.; Hailes, H.C.; Lawrence, M.J.; Tabor, A.B. Gene Delivery Using Ternary Lipopolyplexes Incorporating Branched Cationic Peptides: The Role of Peptide Sequence and Branching. Mol. Pharm. 2013, 10, 127–141. [Google Scholar] [CrossRef]
- Kwok, A.; Eggimann, G.A.; Reymond, J.-L.; Darbre, T.; Hollfelder, F. Peptide Dendrimer/Lipid Hybrid Systems Are Efficient DNA Transfection Reagents: Structure–Activity Relationships Highlight the Role of Charge Distribution Across Dendrimer Generations. ACS Nano 2013, 7, 4668–4682. [Google Scholar] [CrossRef]
- Kwok, A.; Eggimann, G.A.; Heitz, M.; Reymond, J.-L.; Hollfelder, F.; Darbre, T. Efficient Transfection of siRNA by Peptide Dendrimer-Lipid Conjugates. ChemBioChem 2016, 17, 2223–2229. [Google Scholar] [CrossRef] [Green Version]
- Heitz, M.; Javor, S.; Darbre, T.; Reymond, J.-L. Stereoselective pH Responsive Peptide Dendrimers for siRNA Transfection. Bioconjug. Chem. 2019, 30, 2165–2182. [Google Scholar] [CrossRef] [PubMed]
- Delong, R.; Stephenson, K.; Loftus, T.; Fisher, M.; Alahari, S.; Nolting, A.; Juliano, R.L. Characterization of Complexes of Oligonucleotides with Polyamidoamine Starburst Dendrimers and Effects on Intracellular Delivery. J. Pharm. Sci. 1997, 86, 762–764. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, S.; Hughes, M.D.; Khan, A.; Bibby, M.; Hussain, M.; Nawaz, Q.; Double, J.; Sayyed, P. The delivery of antisense therapeutics. Adv. Drug Deliv. Rev. 2000, 44, 3–21. [Google Scholar] [CrossRef]
- Hussain, M.; Shchepinov, M.; Sohail, M.; Benter, I.F.; Hollins, A.J.; Southern, E.M.; Akhtar, S. A novel anionic dendrimer for improved cellular delivery of antisense oligonucleotides. J. Control. Release 2004, 99, 139–155. [Google Scholar] [CrossRef] [PubMed]
- Saher, O.; Rocha, C.S.J.; Zaghloul, E.M.; Wiklander, O.P.B.; Zamolo, S.; Heitz, M.; Ezzat, K.; Gupta, D.; Reymond, J.-L.; Zain, R.; et al. Novel peptide-dendrimer/lipid/oligonucleotide ternary complexes for efficient cellular uptake and improved splice-switching activity. Eur. J. Pharm. Biopharm. 2018, 132, 29–40. [Google Scholar] [CrossRef]
- Delteil, C.; Teissié, J.; Rols, M.-P. Effect of serum on in vitro electrically mediated gene delivery and expression in mammalian cells. Biochim. Biophys. Acta Biomembr. 2000, 1467, 362–368. [Google Scholar] [CrossRef] [Green Version]
- Levine, H.; Slade, L. Another view of trehalose for drying and stabilizing biological materials. BioPharm 1992, 5, 36–40. [Google Scholar]
- Allison, S.D.; Molina, M.d.C.; Anchordoquy, T.J. Stabilization of lipid/DNA complexes during the freezing step of the lyophilization process: The particle isolation hypothesis. Biochim. Biophys. Acta Biomembr. 2000, 1468, 127–138. [Google Scholar] [CrossRef] [Green Version]
- Bestas, B.; Moreno, P.M.D.; Blomberg, K.E.M.; Mohammad, D.K.; Saleh, A.F.; Sutlu, T.; Nordin, J.Z.; Guterstam, P.; Gustafsson, M.O.; Kharazi, S.; et al. Splice-correcting oligonucleotides restore BTK function in X-linked agammaglobulinemia model. J. Clin. Invest. 2014, 124, 4067–4081. [Google Scholar] [CrossRef] [Green Version]
- Kasper, J.C.; Schaffert, D.; Ogris, M.; Wagner, E.; Friess, W. Development of a lyophilized plasmid/LPEI polyplex formulation with long-term stability—A step closer from promising technology to application. J. Control. Release 2011, 151, 246–255. [Google Scholar] [CrossRef]
- Mumper, R.J.; Duguid, J.G.; Anwer, K.; Barron, M.K.; Nitta, H.; Rolland, A.P. Polyvinyl derivatives as novel interactive polymers for controlled gene delivery to muscle. Pharm. Res. 1996, 13, 701–709. [Google Scholar] [CrossRef]
- Kaasalainen, M.; Mäkilä, E.; Riikonen, J.; Kovalainen, M.; Järvinen, K.; Herzig, K.-H.; Lehto, V.-P.; Salonen, J. Effect of isotonic solutions and peptide adsorption on zeta potential of porous silicon nanoparticle drug delivery formulations. Int. J. Pharm. 2012, 431, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Stejskal, J.; Haze, K. [Isotonic solutions]. Cesk. Farm. 1954, 3, 100–104. [Google Scholar]
- Peskin, A.V.; Winterbourn, C.C. A microtiter plate assay for superoxide dismutase using a water-soluble tetrazolium salt (WST-1). Clin. Chim. Acta 2000, 293, 157–166. [Google Scholar] [CrossRef]
- Kang, S.-H.; Cho, M.-J.; Kole, R. Up-Regulation of Luciferase Gene Expression with Antisense Oligonucleotides: Implications and Applications in Functional Assay Development †. Biochemistry 1998, 37, 6235–6239. [Google Scholar] [CrossRef]
- Dominski, Z.; Kole, R. Restoration of correct splicing in thalassemic pre-mRNA by antisense oligonucleotides. Proc. Natl. Acad. Sci. 1993, 90, 8673–8677. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Li, S.; Tan, Y.; Stolz, D.B.; Watkins, S.C.; Block, L.H.; Huang, L. Lyophilization of Cationic Lipid–protamine–DNA (LPD) Complexes. J. Pharm. Sci. 2000, 89, 355–364. [Google Scholar] [CrossRef]
- Boge, L.; Västberg, A.; Umerska, A.; Bysell, H.; Eriksson, J.; Edwards, K.; Millqvist-Fureby, A.; Andersson, M. Freeze-dried and re-hydrated liquid crystalline nanoparticles stabilized with disaccharides for drug-delivery of the plectasin derivative AP114 antimicrobial peptide. J. Colloid Interface Sci. 2018, 522, 126–135. [Google Scholar] [CrossRef]
- Ghanbarzadeh, S.; Valizadeh, H.; Zakeri-Milani, P. The effects of lyophilization on the physico-chemical stability of sirolimus liposomes. Adv. Pharm. Bull. 2013, 3, 25–29. [Google Scholar]
- Zhao, Q.; Temsamani, J.; Agrawal, S. Use of cyclodextrin and its derivatives as carriers for oligonucleotide delivery. Antisense Res. Dev. 1995, 5, 185–192. [Google Scholar] [CrossRef]
- Nair, J.K.; Willoughby, J.L.S.; Chan, A.; Charisse, K.; Alam, M.R.; Wang, Q.; Hoekstra, M.; Kandasamy, P.; Kel’in, A.V.; Milstein, S.; et al. Multivalent N-Acetylgalactosamine-Conjugated siRNA Localizes in Hepatocytes and Elicits Robust RNAi-Mediated Gene Silencing. J. Am. Chem. Soc. 2014, 136, 16958–16961. [Google Scholar] [CrossRef] [Green Version]
- Smith, C.I.E.; Zain, R. Therapeutic Oligonucleotides: State of the Art. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 605–630. [Google Scholar] [CrossRef] [PubMed]
- Kerwin, B.A. Polysorbates 20 and 80 Used in the Formulation of Protein Biotherapeutics: Structure and Degradation Pathways. J. Pharm. Sci. 2008, 97, 2924–2935. [Google Scholar] [CrossRef] [PubMed]
- Wang, W. Lyophilization and development of solid protein pharmaceuticals. Int. J. Pharm. 2000, 203, 1–60. [Google Scholar] [CrossRef]
- Ciftci, K.; Levy, R.J. Enhanced plasmid DNA transfection with lysosomotropic agents in cultured fibroblasts. Int. J. Pharm. 2001, 218, 81–92. [Google Scholar] [CrossRef]
- Gombotz, W.R.; Pankey, S.C.; Phan, D.; Drager, R.; Donaldson, K.; Antonsen, K.P.; Hoffman, A.S.; Raff, H.V. The stabilization of a human IgM monoclonal antibody with poly(vinylpyrrolidone). Pharm. Res. 1994, 11, 624–632. [Google Scholar] [CrossRef]
- Dékány, G.; Csóka, I.; Erös, I. Interaction Between Liposomes and Neutral Polymers: Effect of Adsorption on Drug Release. J. Dispers. Sci. Technol. 2001, 22, 461–472. [Google Scholar] [CrossRef]
- Labidi, N.S.; Djebaili, A. Studies of The Mechanism of Polyvinyl Alcohol Adsorption on The Calcite/Water Interface in The Presence of Sodium Oleate. J. Miner. Mater. Charact. Eng. 2008, 07, 147–161. [Google Scholar] [CrossRef]
- Rubin, A.; Springer, G.F.; Hogue, M.J. The effect of D-glucosamine hydrochloride and related compounds on tissue cultures of the solid form of mouse sarcoma 37. Cancer Res. 1954, 14, 456–458. [Google Scholar]
- Vasir, J.K.; Labhasetwar, V. Biodegradable nanoparticles for cytosolic delivery of therapeutics. Adv. Drug Deliv. Rev. 2007, 59, 718–728. [Google Scholar] [CrossRef] [Green Version]
- Sumner, S.G.; Pringle, I.A.; Gill, D.R.; Hyde, S.C. Effect of Residual Polyvinyl Alcohol on Nanoparticle-Mediated Gene Transfection in Breast Cancer Cells. Mol. Ther. 2003, 7, S67. [Google Scholar]
- Wiśniewska, M.; Ostolska, I.; Szewczuk-Karpisz, K.; Chibowski, S.; Terpiłowski, K.; Gun’ko, V.M.; Zarko, V.I. Investigation of the polyvinyl alcohol stabilization mechanism and adsorption properties on the surface of ternary mixed nanooxide AST 50 (Al2O3–SiO2–TiO2). J. Nanoparticle Res. 2015, 17, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocha, C.S.J.; Lundin, K.E.; Behlke, M.A.; Zain, R.; EL Andaloussi, S.; Smith, C.E. Four Novel Splice-Switch Reporter Cell Lines: Distinct Impact of Oligonucleotide Chemistry and Delivery Vector on Biological Activity. Nucleic Acid Ther. 2016, 26, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Vincent, B. The effect of adsorbed polymers on dispersion stability. Adv. Colloid Interface Sci. 1974, 4, 193–277. [Google Scholar] [CrossRef]
- Ezzat, K.; Helmfors, H.; Tudoran, O.; Juks, C.; Lindberg, S.; Padari, K.; El-Andaloussi, S.; Pooga, M.; Langel, Ü. Scavenger receptor-mediated uptake of cell-penetrating peptide nanocomplexes with oligonucleotides. FASEB J. 2012, 26, 1172–1180. [Google Scholar] [CrossRef]
- Vader, P.; van der Aa, L.J.; Engbersen, J.F.J.; Storm, G.; Schiffelers, R.M. Physicochemical and Biological Evaluation of siRNA Polyplexes Based on PEGylated Poly(amido amine)s. Pharm. Res. 2012, 29, 352–361. [Google Scholar] [CrossRef] [Green Version]
- Kircheis, R.; Wightman, L.; Schreiber, A.; Robitza, B.; Rössler, V.; Kursa, M.; Wagner, E. Polyethylenimine/DNA complexes shielded by transferrin target gene expression to tumors after systemic application. Gene Ther. 2001, 8, 28–40. [Google Scholar] [CrossRef] [Green Version]
- Arms, L.; Smith, D.W.; Flynn, J.; Palmer, W.; Martin, A.; Woldu, A.; Hua, S. Advantages and Limitations of Current Techniques for Analyzing the Biodistribution of Nanoparticles. Front. Pharmacol. 2018, 9, 802. [Google Scholar] [CrossRef]
- Lesniak, W.G.; Mishra, M.K.; Jyoti, A.; Balakrishnan, B.; Zhang, F.; Nance, E.; Romero, R.; Kannan, S.; Kannan, R.M. Biodistribution of Fluorescently Labeled PAMAM Dendrimers in Neonatal Rabbits: Effect of Neuroinflammation. Mol. Pharm. 2013, 10, 4560–4571. [Google Scholar] [CrossRef]









| Sugar Excipients | Final Concentration (w/v) * |
|---|---|
| Sucrose | 9.25% |
| Glucose | 5% |
| Lactose | 9.75% |
| Arabinose | 10% |
| Glucosamine | 5% |
| Galactosamine | 5% |
| N-acetyl Galactosamine | 5% |
| Galactose | 5% |
| Fructose | 5% |
| (2-Hydroxypropyl)-β-cyclodextrin | 10% HP-β-CD + 6.5% sucrose |
| Polymer Excipients | Composition and Final Concentration (w/v) * |
| PVP 10 | 5% PVP 10 + 6.3% sucrose |
| PVP 40 | 5% PVP 40 + 6.3% sucrose |
| PVP 55 | 5% PVP 55 + 6.3% sucrose |
| PVA 18 | 2% PVA 18 + 6.5% sucrose |
| PVA 40 | 2% PVA 40 + 6.5% sucrose |
| Tw 80 | 0.02% Tw80 + 9.25% sucrose |
| Tw 20 | 0.02% Tw20 + 9.25% sucrose |
| PF 68 | 10% PF68 |
| PF 127 | 10% PF127 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saher, O.; Lehto, T.; Gissberg, O.; Gupta, D.; Gustafsson, O.; Andaloussi, S.E.; Darbre, T.; Lundin, K.E.; Smith, C.I.E.; Zain, R. Sugar and Polymer Excipients Enhance Uptake and Splice-Switching Activity of Peptide-Dendrimer/Lipid/Oligonucleotide Formulations. Pharmaceutics 2019, 11, 666. https://doi.org/10.3390/pharmaceutics11120666
Saher O, Lehto T, Gissberg O, Gupta D, Gustafsson O, Andaloussi SE, Darbre T, Lundin KE, Smith CIE, Zain R. Sugar and Polymer Excipients Enhance Uptake and Splice-Switching Activity of Peptide-Dendrimer/Lipid/Oligonucleotide Formulations. Pharmaceutics. 2019; 11(12):666. https://doi.org/10.3390/pharmaceutics11120666
Chicago/Turabian StyleSaher, Osama, Taavi Lehto, Olof Gissberg, Dhanu Gupta, Oskar Gustafsson, Samir EL Andaloussi, Tamis Darbre, Karin E. Lundin, C. I. Edvard Smith, and Rula Zain. 2019. "Sugar and Polymer Excipients Enhance Uptake and Splice-Switching Activity of Peptide-Dendrimer/Lipid/Oligonucleotide Formulations" Pharmaceutics 11, no. 12: 666. https://doi.org/10.3390/pharmaceutics11120666