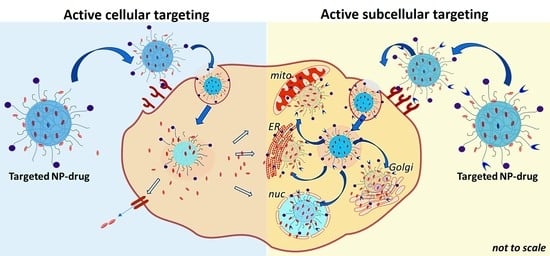
Active Cellular and Subcellular Targeting of Nanoparticles for Drug Delivery
Abstract
:1. Introduction
2. Active Cellular Targeting of Nanoparticle (NP)-Drug Systems
3. Active Subcellular Targeting of NP-Drug Systems
3.1. Targeting of NP-Drug Systems to the Nucleus
3.2. Targeting of NP-Drug Systems to the Mitochondria
4. Conclusions and Future Perspective
Funding
Conflicts of Interest
Appendix A
| NPs/(Avg. Size, nm) | Drug (s) | Surface Coating | Targeting Moieties | Targets/Interaction Mechanism | Diseases/Therapy | NPs Actuation | Cell type/Animal Model | Change in IC50 a | Targeted Accum b | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Liposome (~130) | DOX, IR780 | PEG | FA | FAR/receptor-mediated | Lung cancer/chemo | Laser irradiation | KB and A549/mice | 1.6× (KB cells) | 12.7-fold | [155] |
| Liposome (~100) | DOX | PEG | Anti-HER2 antibody & TZM | HER2/antibody-antigen & ligand | Breast & gastric cancer/chemo | Passive | BT474-M3, NCI-N87/mice | 2-fold | [156] | |
| Liposome (~200) | DOX | PEG | R8 & Tf | TfR/charge- & receptor-mediated | Ovarian cancer/chemo | Passive | A2780/mice | 2-fold | [38] | |
| Liposome (~100) | PTX | PEG | Anti-PD-L1 antibody | PD-L1/antibody-antigen | Lung & colon cancer metastases/chemo | Passive | 4T1, CT26/mice | 2-fold | [37] | |
| Liposome (~120) | SOD, PTX | PEG | RGD | αvβ3 integrin/receptor-mediated | Colon cancer/chemo | Intracellular pH ~ 5.0 | Colon26/mice | 3.4-fold | [157] | |
| Liposome (~100) | PTX, MATT | PEG | HA | CD44/receptor-mediated | Lung cancer metastases/chemo | Passive | 4T1/mice | 1.6-fold | [40] | |
| PEG G2 dendrimers (~200) | PTX, TR3 siRNA | Peptide, KTLLPTP | Plectin-1/peptide-based | Pancreatic cancer/chemo & gene | Intracellular reduction | Panc-1/mice | [55] | |||
| Pullulan (130–170) | PTX | Pullulan & FA | ASGPR & FAR/receptor-mediated | Liver cancer/chemo | Intracellular reduction | SMMC-7721, A549/rats, mice | 3-fold | [59] | ||
| PEG-PTMBPEC (160–180) | DOX | PEG | Peptide, cRGDyK | αvβ3 integrin/receptor-mediated | Skin cancer/chemo | Intracellular pH ~ 5.0 | B16, HUVEC/mice | [60] | ||
| HA-LLA (152–219) | DOX | HA | HA | CD44/receptor-mediated | Breast cancer/chemo | Intracellular reduction | MCF-7/ADR e/mice | 1.17×–4.5× | 20-fold | [61] |
| PHIS (~150) | DOX, R848 | HA | HA | CD44/receptor-mediated | Breast cancer/immuno & chemo | Intracellular pH ~ 5.5) | MCF-7, MDA-MB-231, 4T1/rats, mice | 2.8×–3.8× (4T1 cells) | [64] | |
| Polyaniline (~190) | MTX | PEG | LT | SST receptor/receptor-mediated | Breast cancer/chemo | Intracellular pH ~ 5.0 and laser irradiation | MCF-7, MDA-MB-231 | 0.14× (MCF-7 cells) | 2-fold | [70] |
| Polystyrene (~200) | DTX, GQD | RBC membrane | Ct | EGFR/receptor-mediated | Lung cancer/chemo & PTT | Laser irradiation | A549/mice | 8-fold | [86] | |
| UCNP (~80) | mTHPC, IR-780 | PEG | Angiopep-2 c | Angiopep-2 receptor/receptor-mediated | GBM/PDT & PTT | Laser irradiation | ALTS1C1/mice | [95] | ||
| InP/ZnS QDs (~46) | AF | PEG | Anti-EGFR nanobody | EGFR/antibody-antigen | Breast cancer/chemo | MDA-MB-468/mice | 67-fold | [77] | ||
| PEG-PAE (200) | PTX | Macrophage membrane | Peptide, CSKC | IGFIR/receptor-mediated | Breast cancer/chemo | Intracellular pH ~ 5.0 | MDA-MB-231/mice | 1.5-fold | [66] | |
| Mannosylated albumin (140) | DSF/Cu, Rego | Mannose | MR/receptor-mediated | Colon cancer/chemo | HCT8/ADR f, M2 macrophage/mice | 0.4× | 3-fold | [158] | ||
| HA nanogels (~165) | GrB | Peptide d & HA | EGFR & CD44/receptor-mediated | Ovarian & breast cancer/protein | SKOV-3, MDA-MB-231/mice | 6-fold | [159] | |||
| Polysaccharide (~175) | MTX | Dextran sulfate | Dextran | SR-A/receptor-mediated | RA | RAW264, BAECs/mice | 12-fold | [160] | ||
| PLGA/Fe/Gold (~135) | MTX, AuNP | PEG | Peptide, RGD | αvβ3 integrin/receptor-mediated | RA/chemo & PTT | Laser irradiation | mice | [104] | ||
| P(HDCA-co-MePEGCA) (~125) | PEG | Anti-Aβ1-42 antibody | Amyloid-β peptide/antibody-antigen | AD | Mice | [161] | ||||
| Gold nanorods (GNRs) (~50) | APH | CTAB | Anti-Aβ scFv 12B4 antibody | Aβ aggregation/antibody-antigen | AD/PTT | Laser irradiation | SH-SY5Y/C. elegans model of AD | [118] |
| NPs/(Avg. Size, nm) | Drug (s) | Surface Coating | Targeting Moieties | Cellular Targets/Interaction Mechanism | Subcellular Targets/Interaction Mechanism | Diseases/Therapy | NPs Actuation | Cell type/Animal Model | Change in IC50 a | Targeted Accum b | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MSN (~80) | DOX | FA & DEX | FAR/receptor-mediated | Nucleus/GR receptor-mediated | Cancer/chemo | Passive | HeLa | 0.5× | [125] | ||
| MSN (~40) | DOX | PEG | GAL & TAT | ASGPR/receptor-mediated | Nucleus/NLS | Liver cancer/chemo | Intracellular pH ~ 5.0 | QGY-7703/mice | ~0.5× | 10–40 folds | [133] |
| CuS@MSN (~40) | APS | RGD, TAT | αvβ3 integrin/receptor-mediated | Nucleus/NLS | Recurrence Cancer/PTT | Laser irradiation | HeLa/mice | [162] | |||
| PEG-BIL (~150) | Ir(III) | PEG | FA, oligo-L-lysine | FAR/receptor-mediated | Nucleus/NLS | Cancer | Intracellular pH ~ 5.0 | HeLa, A549/mice | 0.05× | [135] | |
| Fe3O4@MSNs (~90) | TPZ | PEG | Anti-CD13, TAT | CD12/receptor-mediated | Nucleus/NLS | Breast cancer/PPT & chemo | Intracellular hypoxia & Extracellular AMF | BCSCs, MCF-7/mice | 4-fold | [136] | |
| Liposome (~120) | PTX, LND | PEG | HA & TPGS | CD44/receptor-mediated | Mitochondria/ligand-based | Breast cancer/chemo | Passive | MCF-7/MDR e/rats & mice | 0.006× | ~3-fold | [143] |
| AuNS (~95) | DOX, TPP-KLA c | HA | HA & R8 | CD44/receptor-mediated | Mitochondria/MTS | Breast cancer/PTT & chemo | Intracellular enzyme | MCF-7/ADR e/mice | [147] | ||
| ND (~280) | DOX | PEG | FA & MLS peptide d | FAR/receptor-mediated | Mitochondria/MLS | Breast cancer/chemo | Passive | MCF-7/ADR | [148] | ||
| PR (~100) | DOX | FA & DQA | FAR/receptor-mediated | Mitochondria/ligand-based | Breast cancer/chemo | Passive | MCF-7/ADR/mice | 0.31× | ~3-fold | [163] | |
| HS-CAT (~100) | Ce6 | PEG | Anti-PD-L1 antibody & CTPP | PD-L1/antibody-antigen | Mitochondria/ligand-based | Breast cancer/PDT | Intracellular pH ~ 5.0 | 4T1/mice | ~2-fold | [152] | |
| GO (~200) | DOX | PEG | GA | GAR/receptor-mediated | Mitochondria/GA receptor-mediated | Liver cancer/chemo | Passive | HepG2/mice | 0.46× | 13-fold | [153] |
References
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, M.; Azadi, A.; Rafiei, P.; Ashrafi, H. A pharmacokinetic overview of Nanotechnology-Based drug delivery systems: An ADME-oriented approach. Acc. Chem. Res. 2013, 30, 435–467. [Google Scholar] [CrossRef]
- Bae, Y.H.; Park, K. Targeted drug delivery to tumors: Myths, reality and possibility. J. Control. Release 2011, 153, 198–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, R.; Wei, T.; Goldberg, H.; Wang, W.; Cullion, K.; Kohane, D.S. Getting drugs across biological barriers. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Bender, A.; Scheiber, J.; Glick, M.; Davies, J.W.; Azzaoui, K.; Hamon, J.; Urban, L.; Whitebread, S.; Jenkins, J.L. Analysis of pharmacology data and the prediction of adverse drug reactions and off-Target effects from chemical structure. ChemMedChem 2007, 2, 861–873. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. An overview of clinical and commercial impact of drug delivery systems. J. Control. Release 2014, 190, 15–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Say, K.M.; El-Sawy, H.S. Polymeric nanoparticles: Promising platform for drug delivery. Int. J. Pharm. 2017, 528, 675–691. [Google Scholar] [CrossRef] [PubMed]
- Brannon-Peppas, L.; Blanchette, J.O. Nanoparticle and targeted systems for cancer therapy. Adv. Drug Deliv. Rev. 2012, 64, 206–212. [Google Scholar] [CrossRef]
- Saraiva, C.; Praça, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernardino, L. Nanoparticle-Mediated brain drug delivery: Overcoming Blood–Brain barrier to treat neurodegenerative diseases. J. Control. Release 2016, 235, 34–47. [Google Scholar] [CrossRef]
- Koo, O.M.; Rubinstein, I.; Onyuksel, H. Role of nanotechnology in targeted drug delivery and imaging: A concise review. Nanomedicine 2005, 1, 193–212. [Google Scholar] [CrossRef]
- Yu, X.; Trase, I.; Ren, M.; Duval, K.; Guo, X.; Chen, Z. Design of Nanoparticle-Based carriers for targeted drug delivery. J. Nanomater. 2016, 2016, 15. [Google Scholar] [CrossRef] [PubMed]
- Sangtani, A.; Nag, O.K.; Field, L.D.; Breger, J.C.; Delehanty, J.B. Multifunctional nanoparticle composites: Progress in the use of soft and hard nanoparticles for drug delivery and imaging. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1466. [Google Scholar] [CrossRef] [PubMed]
- Nag, O.K.; Field, L.D.; Chen, Y.; Sangtani, A.; Breger, J.C.; Delehanty, J.B. Controlled actuation of therapeutic nanoparticles: An update on recent progress. Ther. Deliv. 2016, 7, 335–352. [Google Scholar] [CrossRef] [PubMed]
- Nag, O.K.; Awasthi, V. Surface engineering of liposomes for stealth behavior. Pharmaceutics 2013, 5, 542–569. [Google Scholar] [CrossRef]
- Field, L.D.; Nag, O.K.; Sangtani, A.; Burns, K.E.; Delehanty, J.B. The role of nanoparticles in the improvement of systemic anticancer drug delivery. Ther. Deliv. 2018, 9, 527–545. [Google Scholar] [CrossRef]
- Mirza, A.Z.; Siddiqui, F.A. Nanomedicine and drug delivery: A mini review. Int. Nano Lett. 2014, 4, 94. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Cho, K.; Wang, X.; Nie, S.; Chen, Z.; Shin, D.M. Therapeutic nanoparticles for drug delivery in cancer. Clin. Cancer Res. 2008, 14, 1310–1316. [Google Scholar] [CrossRef]
- Prabhakar, U.; Maeda, H.; Jain, R.K.; Sevick-Muraca, E.M.; Zamboni, W.; Farokhzad, O.C.; Barry, S.T.; Gabizon, A.; Grodzinski, P.; Blakey, D.C. Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res. 2013, 73, 2412–2417. [Google Scholar] [CrossRef]
- Kobayashi, H.; Watanabe, R.; Choyke, P.L. Improving conventional enhanced permeability and retention (EPR) effects; what is the appropriate target? Theranostics 2013, 4, 81–89. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Singh, R.; Lillard, J.W., Jr. Nanoparticle-Based targeted drug delivery. Exp. Mol. Pathol. 2009, 86, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Bazak, R.; Houri, M.; El Achy, S.; Kamel, S.; Refaat, T. Cancer active targeting by nanoparticles: A comprehensive review of literature. J. Cancer 2015, 141, 769–784. [Google Scholar]
- Gao, P.; Pan, W.; Li, N.; Tang, B. Boosting cancer therapy with Organelle-Targeted nanomaterials. ACS Appl. Mater. Interfaces 2019, 11, 26529–26558. [Google Scholar] [CrossRef]
- Zheng, C.J.; Han, L.Y.; Yap, C.W.; Ji, Z.L.; Cao, Z.W.; Chen, Y.Z. Therapeutic targets: Progress of their exploration and investigation of their characteristics. Pharmacol. Rev. 2006, 58, 259–279. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Colotta, F.; Allavena, P.; Sica, A.; Garlanda, C.; Mantovani, A. Cancer-Related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis 2009, 30, 1073–1081. [Google Scholar] [CrossRef]
- Rosenblum, D.; Joshi, N.; Tao, W.; Karp, J.M.; Peer, D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 2018, 9, 1410. [Google Scholar] [CrossRef]
- Bae, Y.H. Drug targeting and tumor heterogeneity. J. Control. Release 2009, 133, 2–3. [Google Scholar] [CrossRef] [Green Version]
- Boonstra, M.C.; de Geus, S.W.L.; Prevoo, H.A.J.M.; Hawinkels, L.J.A.C.; van de Velde, C.J.H.; Kuppen, P.J.K.; Vahrmeijer, A.L.; Sier, C.F.M. Selecting targets for tumor imaging: An overview of Cancer-Associated membrane proteins. Biomark. Cancer 2016, 8, 119–133. [Google Scholar] [CrossRef]
- Masood, F. Polymeric nanoparticles for targeted drug delivery system for cancer therapy. Mater. Sci. Eng. C 2016, 60, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Jahan, S.T.; Sadat, S.M.A.; Walliser, M.; Haddadi, A. Targeted therapeutic nanoparticles: An immense promise to fight against cancer. J. Drug Deliv. 2017, 2017, 9090325. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.K.; Riaz, M.A.; Zhang, X.; Lin, C.; Wong, K.H.; Chen, X.; Zhang, G.; Lu, A.; Yang, Z. Surface functionalization and targeting strategies of liposomes in solid tumor therapy: A review. Int. J. Mol. Sci. 2018, 19, 195. [Google Scholar] [CrossRef] [PubMed]
- Gabizon, A.A.; Shmeeda, H.; Zalipsky, S. Pros and cons of the liposome platform in cancer drug targeting. J. Liposome Res. 2006, 16, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Patil, Y.; Amitay, Y.; Ohana, P.; Shmeeda, H.; Gabizon, A. Targeting of pegylated liposomal Mitomycin-C prodrug to the folate receptor of cancer cells: Intracellular activation and enhanced cytotoxicity. J. Control. Release 2016, 225, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Gazzano, E.; Rolando, B.; Chegaev, K.; Salaroglio, I.C.; Kopecka, J.; Pedrini, I.; Saponara, S.; Sorge, M.; Buondonno, I.; Stella, B.; et al. Folate-Targeted liposomal nitrooxy-doxorubicin: An effective tool against P-Glycoprotein-Positive and folate Receptor-Positive tumors. J. Control. Release 2018, 270, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Liang, X.; Li, Y.; Sun, T.; Xue, H.; Jin, Z.; Tian, J. Liposomal nanohybrid cerasomes targeted to Pd-L1 enable Dual-Modality imaging and improve antitumor treatments. Cancer Lett. 2018, 414, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, P.; Jhaveri, A.; Pattni, B.; Biswas, S.; Torchilin, V. Transferrin and octaarginine modified Dual-Functional liposomes with improved cancer cell targeting and enhanced intracellular delivery for the treatment of ovarian cancer. Drug Deliv. 2018, 25, 517–532. [Google Scholar] [CrossRef]
- Lin, C.; Zhang, X.; Chen, H.; Bian, Z.; Zhang, G.; Riaz, M.K.; Tyagi, D.; Lin, G.; Zhang, Y.; Wang, J.; et al. Dual-Ligand modified liposomes provide effective local targeted delivery of Lung-Cancer drug by antibody and tumor Lineage-Homing Cell-Penetrating peptide. Drug Deliv. 2018, 25, 256–266. [Google Scholar] [CrossRef]
- Lv, Y.; Xu, C.; Zhao, X.; Lin, C.; Yang, X.; Xin, X.; Zhang, L.; Qin, C.; Han, X.; Yang, L.; et al. Nanoplatform assembled from a CD44-Targeted prodrug and smart liposomes for dual targeting of tumor microenvironment and cancer cells. ACS Nano 2018, 12, 1519–1536. [Google Scholar] [CrossRef]
- Ohradanova-Repic, A.; Nogueira, E.; Hartl, I.; Gomes, A.C.; Preto, A.; Steinhuber, E.; Mühlgrabner, V.; Repic, M.; Kuttke, M.; Zwirzitz, A.; et al. Fab antibody Fragment-Functionalized liposomes for specific targeting of antigen-positive cells. Nanomedicine 2018, 14, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Antimisiaris, S.G.; Mourtas, S.; Marazioti, A. Exosomes and Exosome-Inspired vesicles for targeted drug delivery. Pharmaceutics 2018, 10, 218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, L.; Qin, Z.; Hua, S.; Guo, Z.; Chu, C.; Lin, H.; Zhang, Y.; Li, W.; Zhang, X.; et al. Genetically engineered Liposome-Like nanovesicles as active targeted transport platform. Adv. Mater. 2018, 30, 1705350. [Google Scholar] [CrossRef] [PubMed]
- Kamerkar, S.; LeBleu, V.S.; Sugimoto, H.; Yang, S.; Ruivo, C.F.; Melo, S.A.; Lee, J.J.; Kalluri, R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 2017, 546, 498–503. [Google Scholar] [CrossRef]
- Tian, T.; Zhang, H.-X.; He, C.-P.; Fan, S.; Zhu, Y.-L.; Qi, C.; Huang, N.-P.; Xiao, Z.-D.; Lu, Z.-H.; Tannous, B.A.; et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials 2018, 150, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Yuan, D.; Deygen, I.; Klyachko, N.L.; Kabanov, A.V.; Batrakova, E.V. Engineering Macrophage-Derived exosomes for targeted paclitaxel delivery to pulmonary metastases: In vitro and in vivo evaluations. Nanomedicine 2018, 14, 195–204. [Google Scholar] [CrossRef]
- Ulbrich, K.; Holá, K.; Šubr, V.; Bakandritsos, A.; Tuček, J.; Zbořil, R. Targeted drug delivery with polymers and magnetic nanoparticles: Covalent and noncovalent approaches, release control, and clinical studies. Chem. Rev. 2016, 116, 5338–5431. [Google Scholar] [CrossRef]
- Gu, X.; Wei, Y.; Fan, Q.; Sun, H.; Cheng, R.; Zhong, Z.; Deng, C. cRGD-Decorated biodegradable polytyrosine nanoparticles for robust encapsulation and targeted delivery of doxorubicin to colorectal cancer in vivo. J. Control. Release 2019, 301, 110–118. [Google Scholar] [CrossRef]
- Patel, J.; Amrutiya, J.; Bhatt, P.; Javia, A.; Jain, M.; Misra, A. Targeted delivery of monoclonal antibody conjugated docetaxel loaded PLGA nanoparticles into EGFR overexpressed lung tumour cells. J. Microencapsul. 2018, 35, 204–217. [Google Scholar] [CrossRef]
- Bressler, E.M.; Kim, J.; Shmueli, R.B.; Mirando, A.C.; Bazzazi, H.; Lee, E.; Popel, A.S.; Pandey, N.B.; Green, J.J. Biomimetic peptide display from a polymeric nanoparticle surface for targeting and antitumor activity to human Triple-Negative breast cancer cells. J. Biomed. Mater. Res. Part A 2018, 106, 1753–1764. [Google Scholar] [CrossRef]
- Yang, L.; Sun, H.; Liu, Y.; Hou, W.; Yang, Y.; Cai, R.; Cui, C.; Zhang, P.; Pan, X.; Li, X.; et al. Self-Assembled Aptamer-Grafted hyperbranched polymer nanocarrier for targeted and photoresponsive drug delivery. Angew. Chem. Int. Ed. 2018, 57, 17048–17052. [Google Scholar] [CrossRef] [PubMed]
- Khanna, V.; Kalscheuer, S.; Kirtane, A.; Zhang, W.; Panyam, J. Perlecan-Targeted nanoparticles for drug delivery to Triple-Negative breast cancer. Future Drug Discov. 2019, 1, FDD8. [Google Scholar] [CrossRef] [PubMed]
- Canfarotta, F.; Lezina, L.; Guerreiro, A.; Czulak, J.; Petukhov, A.; Daks, A.; Smolinska-Kempisty, K.; Poma, A.; Piletsky, S.; Barlev, N.A. Specific drug delivery to cancer cells with Double-Imprinted nanoparticles against epidermal growth factor receptor. Nano Lett. 2018, 18, 4641–4646. [Google Scholar] [CrossRef] [PubMed]
- Muntimadugu, E.; Kumar, R.; Saladi, S.; Rafeeqi, T.A.; Khan, W. CD44 targeted chemotherapy for Co-Eradication of breast cancer stem cells and cancer cells using polymeric nanoparticles of salinomycin and paclitaxel. Colloids Surf. B Biointerfaces 2016, 143, 532–546. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, H.; Wang, K.; Hu, Q.; Yao, Q.; Shen, Y.; Yu, G.; Tang, G. Targeted Co-Delivery of PTX and TR3 siRNA by ptp peptide modified dendrimer for the treatment of pancreatic cancer. Small 2017, 13, 1602697. [Google Scholar] [CrossRef]
- You, C.; Wu, H.; Wang, M.; Gao, Z.; Sun, B.; Zhang, X. Synthesis and biological evaluation of redox/NIR dual Stimulus-Responsive polymeric nanoparticles for targeted delivery of cisplatin. Mater. Sci. Eng. C 2018, 92, 453–462. [Google Scholar] [CrossRef]
- Venkatesan, P.; Thirumalaivasan, N.; Yu, H.-P.; Lai, P.-S.; Wu, S.-P. Redox stimuli delivery vehicle based on Transferrin-Capped msnps for targeted drug delivery in cancer therapy. ACS Appl. Bio Mater. 2019, 2, 1623–1633. [Google Scholar] [CrossRef]
- Carvalho, A.M.; Teixeira, R.; Novoa-Carballal, R.; Pires, R.A.; Reis, R.L.; Pashkuleva, I. Redox-Responsive micellar nanoparticles from glycosaminoglycans for CD44 targeted drug delivery. Biomacromolecules 2018, 19, 2991–2999. [Google Scholar] [CrossRef]
- Huang, L.; Chaurasiya, B.; Wu, D.; Wang, H.; Du, Y.; Tu, J.; Webster, T.J.; Sun, C. Versatile Redox-Sensitive pullulan nanoparticles for enhanced liver targeting and efficient cancer therapy. Nanomedicine 2018, 14, 1005–1017. [Google Scholar] [CrossRef]
- Qiu, L.; Hu, Q.; Cheng, L.; Li, L.; Tian, C.; Chen, W.; Chen, Q.; Hu, W.; Xu, L.; Yang, J.; et al. cRGDyK modified pH responsive nanoparticles for specific intracellular delivery of doxorubicin. Acta Biomater. 2016, 30, 285–298. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhang, J.; Cheng, R.; Deng, C.; Meng, F.; Xie, F.; Zhong, Z. Reversibly crosslinked hyaluronic acid nanoparticles for active targeting and intelligent delivery of doxorubicin to drug resistant CD44+ human breast tumor xenografts. J. Control. Release 2015, 205, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Rodzinski, A.; Guduru, R.; Liang, P.; Hadjikhani, A.; Stewart, T.; Stimphil, E.; Runowicz, C.; Cote, R.; Altman, N.; Datar, R.; et al. Targeted and controlled anticancer drug delivery and release with magnetoelectric nanoparticles. Sci. Rep. 2016, 6, 20867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niedermayer, S.; Weiss, V.; Herrmann, A.; Schmidt, A.; Datz, S.; Müller, K.; Wagner, E.; Bein, T.; Bräuchle, C. Multifunctional Polymer-Capped mesoporous silica nanoparticles for pH-responsive targeted drug delivery. Nanoscale 2015, 7, 7953–7964. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qiao, L.; Zhang, S.; Wan, G.; Chen, B.; Zhou, P.; Zhang, N.; Wang, Y. Dual pH-Responsive multifunctional nanoparticles for targeted treatment of breast cancer by combining immunotherapy and chemotherapy. Acta Biomater. 2018, 66, 310–324. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Cun, X.; Ruan, S.; Liu, R.; Xiao, W.; Yang, X.; Yang, Y.; Yang, C.; Gao, H. Enzyme-Triggered size shrink and Laser-Enhanced no release nanoparticles for deep tumor penetration and combination therapy. Biomaterials 2018, 168, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cai, K.; Li, C.; Guo, Q.; Chen, Q.; He, X.; Liu, L.; Zhang, Y.; Lu, Y.; Chen, X.; et al. Macrophage-Membrane-Coated nanoparticles for Tumor-Targeted chemotherapy. Nano Lett. 2018, 18, 1908–1915. [Google Scholar] [CrossRef] [PubMed]
- Tseng, S.J.; Kempson, I.M.; Huang, K.-Y.; Li, H.-J.; Fa, Y.-C.; Ho, Y.-C.; Liao, Z.-X.; Yang, P.-C. Targeting tumor microenvironment by Bioreduction-Activated nanoparticles for Light-Triggered virotherapy. ACS Nano 2018, 12, 9894–9902. [Google Scholar] [CrossRef]
- Wang, M.; You, C.; Gao, Z.; Wu, H.; Sun, B.; Zhu, X.; Chen, R. A Dual-Targeting strategy for enhanced drug delivery and synergistic therapy based on thermosensitive nanoparticles. J. Biomater. Sci. Polym. Ed. 2018, 29, 1360–1374. [Google Scholar] [CrossRef]
- Delehanty, J.B.; Medintz, I.L. Controlled actuation of therapeutic nanoparticles: Moving beyond passive delivery modalities. Ther. Deliv. 2013, 4, 127–129. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Phung, C.D.; Thapa, R.K.; Pham, T.T.; Tran, T.H.; Jeong, J.-H.; Ku, S.K.; Choi, H.-G.; Yong, C.S.; Kim, J.O. Multifunctional nanoparticles as somatostatin Receptor-Targeting delivery system of polyaniline and methotrexate for combined Chemo–Photothermal therapy. Acta Biomater. 2018, 68, 154–167. [Google Scholar] [CrossRef]
- Hu, C.; Yang, X.; Liu, R.; Ruan, S.; Zhou, Y.; Xiao, W.; Yu, W.; Yang, C.; Gao, H. Coadministration of iRGD with multistage responsive nanoparticles enhanced tumor targeting and penetration abilities for breast cancer therapy. ACS Appl. Mater. Interfaces 2018, 10, 22571–22579. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Saw, P.E.; Nie, Y.; Wong, P.-P.; Jiang, L.; Ye, X.; Chen, J.; Ding, T.; Xu, L.; Yao, H.; et al. Multifunctional sharp pH-Responsive nanoparticles for targeted drug delivery and effective breast cancer therapy. J. Mater. Chem. B 2019, 7, 576–585. [Google Scholar] [CrossRef]
- Rajendrakumar, S.K.; Cherukula, K.; Park, H.J.; Uthaman, S.; Jeong, Y.Y.; Lee, B.-I.; Park, I.-K. Dual-Stimuli-Responsive Albumin-Polyplex nanoassembly for spatially controlled gene release in metastatic breast cancer. J. Control. Release 2018, 276, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Michalska, M.; Florczak, A.; Dams-Kozlowska, H.; Gapinski, J.; Jurga, S.; Schneider, R. Peptide-Functionalized ZCIS QDs as fluorescent nanoprobe for targeted HER2-Positive breast cancer cells imaging. Acta Biomater. 2016, 35, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Bwatanglang, I.B.; Mohammad, F.; Yusof, N.A.; Abdullah, J.; Hussein, M.Z.; Alitheen, N.B.; Abu, N. Folic acid targeted Mn:Zns quantum dots for theranostic applications of cancer cell imaging and therapy. Int. J. Nanomed. 2016, 11, 413–428. [Google Scholar]
- AbdElhamid, A.S.; Zayed, D.G.; Helmy, M.W.; Ebrahim, S.M.; Bahey-El-Din, M.; Zein-El-Dein, E.A.; El-Gizawy, S.A.; Elzoghby, A.O. Lactoferrin-Tagged quantum dots-based theranostic nanocapsules for combined COX-2 inhibitor/herbal therapy of breast cancer. Nanomedicine 2018, 13, 2637–2656. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Chen, G.; Li, Y.; Xu, W.; Gong, S. Quantum-Dot-Based theranostic micelles conjugated with an Anti-EGFR nanobody for Triple-Negative breast cancer therapy. ACS Appl. Mater. Interfaces 2017, 9, 30297–30305. [Google Scholar] [CrossRef]
- Cai, X.; Luo, Y.; Zhang, W.; Du, D.; Lin, Y. pH-Sensitive zno quantum Dots–Doxorubicin nanoparticles for lung cancer targeted drug delivery. ACS Appl. Mater. Interfaces 2016, 8, 22442–22450. [Google Scholar] [CrossRef]
- Kim, M.W.; Jeong, H.Y.; Kang, S.J.; Jeong, I.H.; Choi, M.J.; You, Y.M.; Im, C.S.; Song, I.H.; Lee, T.S.; Lee, J.S.; et al. Anti-EGF receptor Aptamer-Guided Co-Delivery of Anti-Cancer siRNAs and quantum dots for theranostics of Triple-Negative breast cancer. Theranostics 2019, 9, 837–852. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, F.; Zhao, Y.; Zhang, J.; Dawulieti, J.; Pan, Y.; Cui, L.; Sun, M.; Shao, D.; Li, M.; et al. Self-assembled dual fluorescence nanoparticles for CD44-Targeted delivery of anti-miR-27a in liver cancer theranostics. Theranostics 2018, 8, 3808–3823. [Google Scholar] [CrossRef]
- Liang, Z.; Yang, Y.; Jia, F.; Sai, K.; Ullah, S.; Fidelis, C.; Lin, Z.; Li, F. Intrathecal delivery of folate conjugated Near-Infrared quantum dots for targeted in vivo imaging of gliomas in mice brains. ACS Appl. Bio Mater. 2019, 2, 1432–1439. [Google Scholar] [CrossRef]
- Deng, T.; Peng, Y.; Zhang, R.; Wang, J.; Zhang, J.; Gu, Y.; Huang, D.; Deng, D. Water-Solubilizing hydrophobic ZnAgInSe/ZnS QDs with Tumor-Targeted cRGD-Sulfobetaine-PIMA-Histamine ligands via a Self-Assembly strategy for bioimaging. ACS Appl. Mater. Interfaces 2017, 9, 11405–11414. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Dong, H.; Yang, Z.; Zhong, X.; Chen, Y.; Dai, W.; Zhang, X. Aptamer-Conjugated graphene quantum dots/porphyrin derivative theranostic agent for intracellular Cancer-Related microrna detection and Fluorescence-Guided photothermal/photodynamic synergetic therapy. ACS Appl. Mater. Interfaces 2017, 9, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Deng, S.; Liu, J.; Zhong, X.; He, J.; Chen, X.; Feng, B.; Chen, Y.; Ostrikov, K. Cancer-Targeting graphene quantum dots: Fluorescence quantum yields, stability, and cell selectivity. Adv. Funct. Mater. 2019, 29, 1805860. [Google Scholar] [CrossRef]
- Su, Z.; Shen, H.; Wang, H.; Wang, J.; Li, J.; Nienhaus, G.U.; Shang, L.; Wei, G. Motif-Designed peptide nanofibers decorated with graphene quantum dots for simultaneous targeting and imaging of tumor cells. Adv. Funct. Mater. 2015, 25, 5472–5478. [Google Scholar] [CrossRef]
- Sung, S.-Y.; Su, Y.-L.; Cheng, W.; Hu, P.-F.; Chiang, C.-S.; Chen, W.-T.; Hu, S.-H. Graphene quantum Dots-Mediated theranostic penetrative delivery of drug and photolytics in deep tumors by targeted biomimetic nanosponges. Nano Lett. 2019, 19, 69–81. [Google Scholar] [CrossRef]
- Su, Y.-L.; Yu, T.-W.; Chiang, W.-H.; Chiu, H.-C.; Chang, C.-H.; Chiang, C.-S.; Hu, S.-H. Hierarchically targeted and penetrated delivery of drugs to tumors by Size-Changeable graphene quantum dot nanoaircrafts for photolytic therapy. Adv. Funct. Mater. 2017, 27, 1700056. [Google Scholar] [CrossRef]
- Chen, C.-L.; Siow, T.Y.; Chou, C.-H.; Lin, C.-H.; Lin, M.-H.; Chen, Y.-C.; Hsieh, W.-Y.; Wang, S.-J.; Chang, C. Targeted superparamagnetic iron oxide nanoparticles for in vivo magnetic resonance imaging of T-Cells in rheumatoid arthritis. Mol. Imaging Biol. 2017, 19, 233–244. [Google Scholar] [CrossRef]
- Martínez-Carmona, M.; Lozano, D.; Colilla, M.; Vallet-Regí, M. Lectin-Conjugated pH-Responsive mesoporous silica nanoparticles for targeted bone cancer treatment. Acta Biomater. 2018, 65, 393–404. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, J.; Zhu, W.; Sun, C.; Di, D.; Zhang, Y.; Wang, P.; Wang, Z.; Wang, S. Dual-Stimuli responsive hyaluronic Acid-Conjugated mesoporous silica for targeted delivery to CD44-Overexpressing cancer cells. Acta Biomater. 2015, 23, 147–156. [Google Scholar] [CrossRef]
- Choi, J.Y.; Ramasamy, T.; Kim, S.Y.; Kim, J.; Ku, S.K.; Youn, Y.S.; Kim, J.-R.; Jeong, J.-H.; Choi, H.-G.; Yong, C.S.; et al. PEGylated lipid bilayer-supported mesoporous silica nanoparticle composite for synergistic Co-Delivery of axitinib and celastrol in Multi-Targeted cancer therapy. Acta Biomater. 2016, 39, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Samykutty, A.; Grizzle, W.E.; Fouts, B.L.; McNally, M.W.; Chuong, P.; Thomas, A.; Chiba, A.; Otali, D.; Woloszynska, A.; Said, N.; et al. Optoacoustic imaging identifies ovarian cancer using a microenvironment targeted theranostic wormhole mesoporous silica nanoparticle. Biomaterials 2018, 182, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Coluccia, D.; Figueiredo, C.A.; Wu, M.Y.; Riemenschneider, A.N.; Diaz, R.; Luck, A.; Smith, C.; Das, S.; Ackerley, C.; O’Reilly, M.; et al. Enhancing glioblastoma treatment using Cisplatin-Gold-Nanoparticle conjugates and targeted delivery with magnetic Resonance-Guided focused ultrasound. Nanomedicine 2018, 14, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.; Bu, L.-L.; Xu, J.-H.; Cai, B.; Yu, G.-T.; Yu, X.; He, Z.; Huang, Q.; Li, A.; Guo, S.-S.; et al. Red blood cell membrane as a biomimetic nanocoating for prolonged circulation time and reduced accelerated blood clearance. Small 2015, 11, 6225–6236. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-C.; Vijayaraghavan, P.; Chiang, W.-H.; Chen, H.-H.; Liu, T.-I.; Shen, M.-Y.; Omoto, A.; Kamimura, M.; Soga, K.; Chiu, H.-C. Targeted delivery of functionalized upconversion nanoparticles for externally triggered photothermal/photodynamic therapies of brain glioblastoma. Theranostics 2018, 8, 1435–1448. [Google Scholar] [CrossRef]
- Cheng, W.; Nie, J.; Xu, L.; Liang, C.; Peng, Y.; Liu, G.; Wang, T.; Mei, L.; Huang, L.; Zeng, X. pH-Sensitive delivery vehicle based on folic Acid-Conjugated Polydopamine-Modified mesoporous silica nanoparticles for targeted cancer therapy. ACS Appl. Mater. Interfaces 2017, 9, 18462–18473. [Google Scholar] [CrossRef]
- Pirmardvand Chegini, S.; Varshosaz, J.; Taymouri, S. Recent approaches for targeted drug delivery in rheumatoid arthritis diagnosis and treatment. Artif. Cells Nanomed. Biotechnol. 2018, 46, 502–514. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, M.; Onuoha, S.C.; Pitzalis, C. Trojan horses and guided missiles: Targeted therapies in the war on arthritis. Nat. Rev. Rheumatol. 2015, 11, 328. [Google Scholar] [CrossRef]
- Nogueira, E.; Gomes, A.C.; Preto, A.; Cavaco-Paulo, A. Folate-Targeted nanoparticles for rheumatoid arthritis therapy. Nanomedicine 2016, 12, 1113–1126. [Google Scholar] [CrossRef]
- Chen, M.; Daddy, J.C.K.A.; Xiao, Y.; Ping, Q.; Zong, L. Advanced nanomedicine for rheumatoid arthritis treatment: Focus on active targeting. Expert Opin. Drug Deliv. 2017, 14, 1141–1144. [Google Scholar] [CrossRef]
- Put, S.; Westhovens, R.; Lahoutte, T.; Matthys, P. Molecular imaging of rheumatoid arthritis: Emerging markers, tools, and techniques. Arthritis Res. Ther. 2014, 16, 208. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhao, M.; Yu, C.; Zhang, X.; Liu, J.; Cheng, X.; Lee, R.J.; Sun, F.; Teng, L.; Li, Y. Multifunctional folate Receptor-Targeting and pH-Responsive nanocarriers loaded with methotrexate for treatment of rheumatoid arthritis. Int. J. Nanomed. 2017, 12, 6735–6746. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Li, H. Combination of NF-kB targeted siRNA and methotrexate in a hybrid nanocarrier towards the effective treatment in rheumatoid arthritis. J. Nanobiotechnol. 2018, 16, 58. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, S.-M.; Park, K.-H.; Mun, C.H.; Park, Y.-B.; Yoo, K.-H. Drug-Loaded gold/iron/gold plasmonic nanoparticles for magnetic targeted Chemo-Photothermal treatment of rheumatoid arthritis. Biomaterials 2015, 61, 95–102. [Google Scholar] [CrossRef]
- Lee, S.-M.; Kim, H.J.; Ha, Y.-J.; Park, Y.N.; Lee, S.-K.; Park, Y.-B.; Yoo, K.-H. Targeted Chemo-Photothermal treatments of rheumatoid arthritis using gold Half-Shell multifunctional nanoparticles. ACS Nano 2013, 7, 50–57. [Google Scholar] [CrossRef]
- Costa Lima, S.A.; Reis, S. Temperature-Responsive polymeric nanospheres containing methotrexate and gold nanoparticles: A Multi-Drug system for theranostic in rheumatoid arthritis. Colloids Surf. B Biointerfaces 2015, 133, 378–387. [Google Scholar] [CrossRef]
- Roome, T.; Aziz, S.; Razzak, A.; Aslam, Z.; Lubna; Jamali, K.S.; Sikandar, B.; Fatima, T.; Abidi, L.; Imran, M.; et al. Opuntioside, opuntiol and its metallic nanoparticles attenuate Adjuvant-Induced arthritis: Novel suppressors of Toll-Like receptors -2 and -4. Biomed. Pharmacother. 2019, 112, 108624. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, L.; Cao, J.; Wang, K.; Ge, Y.; Ma, W.; Qi, X.; Shen, S. Effect of magnetic nanoparticles size on rheumatoid arthritis targeting and photothermal therapy. Colloids Surf. B Biointerfaces 2018, 170, 224–232. [Google Scholar] [CrossRef]
- Wen, M.M.; El-Salamouni, N.S.; El-Refaie, W.M.; Hazzah, H.A.; Ali, M.M.; Tosi, G.; Farid, R.M.; Blanco-Prieto, M.J.; Billa, N.; Hanafy, A.S. Nanotechnology-Based drug delivery systems for Alzheimer’s disease management: Technical, industrial, and clinical challenges. J. Control. Release 2017, 245, 95–107. [Google Scholar] [CrossRef]
- Zhou, Y.; Peng, Z.; Seven, E.S.; Leblanc, R.M. Crossing the Blood-Brain barrier with nanoparticles. J. Control. Release 2018, 270, 290–303. [Google Scholar] [CrossRef]
- Clark, A.J.; Davis, M.E. Increased brain uptake of targeted nanoparticles by adding an Acid-Cleavable linkage between transferrin and the nanoparticle core. Proc. Natl. Acad. Sci. USA 2015, 112, 12486–12491. [Google Scholar] [CrossRef] [PubMed]
- Roney, C.; Kulkarni, P.; Arora, V.; Antich, P.; Bonte, F.; Wu, A.; Mallikarjuana, N.N.; Manohar, S.; Liang, H.-F.; Kulkarni, A.R.; et al. Targeted nanoparticles for drug delivery through the Blood–Brain barrier for Alzheimer’s disease. J. Control. Release 2005, 108, 193–214. [Google Scholar] [CrossRef] [PubMed]
- Small, D.H.; McLean, C.A. Alzheimer’s disease and the amyloid β protein. J. Neurochem. 1999, 73, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Sadigh-Eteghad, S.; Sabermarouf, B.; Majdi, A.; Talebi, M.; Farhoudi, M.; Mahmoudi, J. Amyloid-Beta: A crucial factor in Alzheimer’s disease. Med. Prin. Pract. 2015, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ahlschwede, K.M.; Curran, G.L.; Rosenberg, J.T.; Grant, S.C.; Sarkar, G.; Jenkins, R.B.; Ramakrishnan, S.; Poduslo, J.F.; Kandimalla, K.K. Cationic carrier peptide enhances cerebrovascular targeting of nanoparticles in Alzheimer’s disease brain. Nanomedicine 2019, 16, 258–266. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, C.; Guo, Q.; Wan, X.; Shao, X.; Liu, Q.; Zhang, Q. Dual-Functional nanoparticles for precise drug delivery to Alzheimer’s disease lesions: Targeting mechanisms, pharmacodynamics and safety. Int. J. Pharm. 2017, 525, 237–248. [Google Scholar] [CrossRef]
- Lee, H.; Kim, Y.; Park, A.; Nam, J.-M. Amyloid-β aggregation with gold nanoparticles on brain lipid bilayer. Small 2014, 10, 1779–1789. [Google Scholar] [CrossRef]
- Liu, D.; Li, W.; Jiang, X.; Bai, S.; Liu, J.; Liu, X.; Shi, Y.; Kuai, Z.; Kong, W.; Gao, R.; et al. Using Near-Infrared enhanced thermozyme and scFv Dual-Conjugated au nanorods for detection and targeted photothermal treatment of Alzheimer’s disease. Theranostics 2019, 9, 2268–2281. [Google Scholar] [CrossRef]
- Rajendran, L.; Knölker, H.-J.; Simons, K. Subcellular targeting strategies for drug design and delivery. Nat. Rev. Drug Discov. 2010, 9, 29. [Google Scholar] [CrossRef]
- Ma, X.; Gong, N.; Zhong, L.; Sun, J.; Liang, X.-J. Future of nanotherapeutics: Targeting the cellular sub-organelles. Biomaterials 2016, 97, 10–21. [Google Scholar] [CrossRef]
- Sakhrani, N.M.; Padh, H. Organelle targeting: Third level of drug targeting. Drug Des. Dev. Ther. 2013, 7, 585–599. [Google Scholar]
- Torchilin, V.P. Tat Peptide-Mediated intracellular delivery of pharmaceutical nanocarriers. Adv. Drug Deliv. Rev. 2008, 60, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, X.; Yang, H.; Li, L.; Ye, Z.; Rao, Y. A nuclear targeted Dox-Aptamer loaded liposome delivery platform for the circumvention of drug resistance in breast cancer. Biomed. Pharmacother. 2019, 117, 109072. [Google Scholar] [CrossRef] [PubMed]
- Han, S.-S.; Li, Z.-Y.; Zhu, J.-Y.; Han, K.; Zeng, Z.-Y.; Hong, W.; Li, W.-X.; Jia, H.-Z.; Liu, Y.; Zhuo, R.-X.; et al. Dual-pH sensitive Charge-Reversal polypeptide micelles for Tumor-Triggered targeting uptake and nuclear drug delivery. Small 2015, 11, 2543–2554. [Google Scholar] [CrossRef]
- Xiong, L.; Du, X.; Kleitz, F.; Qiao, S.Z. Cancer-Cell-Specific Nuclear-Targeted drug delivery by Dual-Ligand-Modified mesoporous silica nanoparticles. Small 2015, 11, 5919–5926. [Google Scholar] [CrossRef]
- Song, Y.; Tang, C.; Yin, C. Enhanced antitumor efficacy of arginine modified amphiphilic nanoparticles Co-Delivering doxorubicin and iSur-pDNA via the multiple synergistic effect. Biomaterials 2018, 150, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Shen, H.; Zhan, J.; Lin, M.; Dai, L.; Ren, C.; Shi, Y.; Liu, J.; Gao, J.; Yang, Z. Supramolecular “trojan horse” for nuclear delivery of dual anticancer drugs. J. Am. Chem. Soc. 2017, 139, 2876–2879. [Google Scholar] [CrossRef]
- Hua, Q.; Qiang, Z.; Chu, M.; Shi, D.; Ren, J. Polymeric drug delivery system with actively targeted cell penetration and nuclear targeting for cancer therapy. ACS Appl. Bio Mater. 2019, 2, 1724–1731. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, X.; Guo, Y.; Zhu, Y.-X.; Liu, X.; Chen, Z.; Wu, F.-G. Smart supramolecular “trojan horse”—Inspired nanogels for realizing Light-Triggered nuclear drug influx in Drug-Resistant cancer cells. Adv. Funct. Mater. 2019, 29, 1807772. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, L.; Gao, H.; Liu, Y.; Zhang, Q.; Ran, R.; Zhang, Z.; He, Q. Co-Delivery of doxorubicin and P-gp inhibitor by a Reduction-Sensitive liposome to overcome multidrug resistance, enhance Anti-Tumor efficiency and reduce toxicity. Drug Deliv. 2016, 23, 1130–1143. [Google Scholar] [CrossRef]
- Lu, Y.-L.; Ma, Y.-B.; Feng, C.; Zhu, D.-L.; Liu, J.; Chen, L.; Liang, S.-J.; Dong, C.-Y. Co-Delivery of cyclopamine and doxorubicin mediated by bovine serum albumin nanoparticles reverses doxorubicin resistance in breast cancer by down-regulating P-Glycoprotein expression. J. Cancer 2019, 10, 2357–2368. [Google Scholar] [CrossRef] [PubMed]
- Modok, S.; Mellor, H.R.; Callaghan, R. Modulation of multidrug resistance efflux pump activity to overcome chemoresistance in cancer. Curr. Opin. Pharmacol. 2006, 6, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Tang, C.; Yin, C. pH-Responsive Core–Shell structured nanoparticles for triple-stage targeted delivery of doxorubicin to tumors. ACS Appl. Mater. Interfaces 2016, 8, 23498–23508. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Tang, J.; Zheng, R.; Guo, G.; Dong, A.; Wang, Y.; Yang, W. Nuclear-Targeted multifunctional magnetic nanoparticles for photothermal therapy. Adv. Healthc. Mater. 2017, 6, 1601289. [Google Scholar] [CrossRef]
- Fan, Y.; Li, C.; Li, F.; Chen, D. pH-Activated size reduction of large compound nanoparticles for in vivo Nucleus-Targeted drug delivery. Biomaterials 2016, 85, 30–39. [Google Scholar] [CrossRef]
- Li, H.; Yan, W.; Suo, X.; Peng, H.; Yang, X.; Li, Z.; Zhang, J.; Liu, D. Nucleus-Targeted nano delivery system eradicates cancer stem cells by combined thermotherapy and Hypoxia-Activated chemotherapy. Biomaterials 2019, 200, 1–14. [Google Scholar] [CrossRef]
- Smith, R.A.; Hartley, R.C.; Murphy, M.P. Mitochondria-Targeted small molecule therapeutics and probes. Antioxid. Redox Signal. 2011, 15, 3021–3038. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A. A review on mitochondrial restorative mechanism of antioxidants in Alzheimer’s disease and other neurological conditions. Front. Pharmacol. 2015, 6. [Google Scholar] [CrossRef]
- Hou, X.-S.; Wang, H.-S.; Mugaka, B.P.; Yang, G.-J.; Ding, Y. Mitochondria: Promising organelle targets for cancer diagnosis and treatment. Biomater. Sci. 2018, 6, 2786–2797. [Google Scholar] [CrossRef]
- Wang, H.; Gao, Z.; Liu, X.; Agarwal, P.; Zhao, S.; Conroy, D.W.; Ji, G.; Yu, J.; Jaroniec, C.P.; Liu, Z.; et al. Targeted production of reactive oxygen species in mitochondria to overcome cancer drug resistance. Nat. Commun. 2018, 9, 562. [Google Scholar] [CrossRef]
- Battogtokh, G.; Cho, Y.-Y.; Lee, J.Y.; Lee, H.S.; Kang, H.C. Mitochondrial-Targeting anticancer agent conjugates and nanocarrier systems for cancer treatment. Front. Pharmacol. 2018, 9, 922. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-N.; Guo, N.-N.; Guo, W.-W.; Huang-Fu, M.-Y.; Vakili, M.R.; Chen, J.-J.; Xu, W.-H.; Wei, Q.-C.; Han, M.; Lavasanifar, A.; et al. Delivery of mitochondriotropic doxorubicin derivatives using Self-Assembling hyaluronic acid nanocarriers in Doxorubicin-Resistant breast cancer. Acta Pharmacol. Sin. 2018, 39, 1681–1692. [Google Scholar] [CrossRef] [PubMed]
- Assanhou, A.G.; Li, W.; Zhang, L.; Xue, L.; Kong, L.; Sun, H.; Mo, R.; Zhang, C. Reversal of multidrug resistance by Co-Delivery of paclitaxel and lonidamine using a TPGS and hyaluronic acid Dual-Functionalized liposome for cancer treatment. Biomaterials 2015, 73, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Li, L.; He, X.; Yi, Q.; He, B.; Cao, J.; Pan, W.; Gu, Z. Overcoming Drug-Resistant lung cancer by paclitaxel loaded dual-functional liposomes with mitochondria targeting and pH-Response. Biomaterials 2015, 52, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Field, L.D.; Delehanty, J.B.; Chen, Y.; Medintz, I.L. Peptides for specifically targeting nanoparticles to cellular organelles: Quo vadis? Acc. Chem. Res. 2015, 48, 1380–1390. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, E.; Yamada, Y.; Yasuzaki, Y.; Hyodo, M.; Harashima, H. Intracellular observation of nanocarriers modified with a mitochondrial targeting signal peptide. J. Biosci. Bioeng. 2013, 116, 634–637. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Lei, Q.; Qiu, W.-X.; Liu, L.-H.; Zheng, D.-W.; Fan, J.-X.; Rong, L.; Sun, Y.-X.; Zhang, X.-Z. Mitochondria-Targeting “nanoheater” for enhanced photothermal/Chemo-Therapy. Biomaterials 2017, 117, 92–104. [Google Scholar] [CrossRef]
- Chan, M.S.; Liu, L.S.; Leung, H.M.; Lo, P.K. Cancer-Cell-Specific Mitochondria-Targeted drug delivery by Dual-Ligand-Functionalized nanodiamonds circumvent drug resistance. ACS Appl. Mater. Interfaces 2017, 9, 11780–11789. [Google Scholar] [CrossRef]
- Marrache, S.; Dhar, S. Engineering of blended nanoparticle platform for delivery of Mitochondria-Acting therapeutics. Proc. Natl. Acad. Sci. USA 2012, 109, 16288–16293. [Google Scholar] [CrossRef]
- Ding, H.; Lv, Y.; Ni, D.; Wang, J.; Tian, Z.; Wei, W.; Ma, G. Erythrocyte Membrane-Coated NIR-Triggered biomimetic nanovectors with programmed delivery for photodynamic therapy of cancer. Nanoscale 2015, 7, 9806–9815. [Google Scholar] [CrossRef]
- Song, Y.; Shi, Q.; Zhu, C.; Luo, Y.; Lu, Q.; Li, H.; Ye, R.; Du, D.; Lin, Y. Mitochondrial-Targeted multifunctional mesoporous Au@Pt nanoparticles for Dual-Mode photodynamic and photothermal therapy of cancers. Nanoscale 2017, 9, 15813–15824. [Google Scholar] [CrossRef]
- Yang, G.; Xu, L.; Xu, J.; Zhang, R.; Song, G.; Chao, Y.; Feng, L.; Han, F.; Dong, Z.; Li, B.; et al. Smart nanoreactors for pH-Responsive tumor homing, Mitochondria-Targeting, and enhanced Photodynamic-Immunotherapy of cancer. Nano Lett. 2018, 18, 2475–2484. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Z.; Zheng, Y.; Geng, Y.; Han, C.; Shi, Y.; Sun, H.; Zhang, C.; Chen, Y.; Zhang, L.; et al. Glycyrrhetinic acid functionalized graphene oxide for mitochondria targeting and cancer treatment in vivo. Small 2018, 14. [Google Scholar] [CrossRef]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based medicines: A review of FDA-Approved materials and clinical trials to date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Guo, F.; Yu, M.; Wang, J.; Tan, F.; Li, N. Smart IR780 theranostic nanocarrier for Tumor-Specific therapy: Hyperthermia-Mediated Bubble-Generating and Folate-Targeted liposomes. ACS Appl. Mater. Interfaces 2015, 7, 20556–20567. [Google Scholar] [CrossRef]
- Espelin, C.W.; Leonard, S.C.; Geretti, E.; Wickham, T.J.; Hendriks, B.S. Dual HER2 targeting with trastuzumab and liposomal-encapsulated doxorubicin (MM-302) demonstrates synergistic antitumor activity in breast and gastric cancer. Cancer Res. 2016, 76, 1517–1527. [Google Scholar] [CrossRef]
- Peng, J.Q.; Fumoto, S.; Suga, T.; Miyamoto, H.; Kuroda, N.; Kawakami, S.; Nishida, K. Targeted Co-Delivery of protein and drug to a tumor in vivo by sophisticated RGD-modified Lipid-Calcium carbonate nanoparticles. J. Control. Release 2019, 302, 42–53. [Google Scholar] [CrossRef]
- Zhao, P.; Yin, W.; Wu, A.; Tang, Y.; Wang, J.; Pan, Z.; Lin, T.; Zhang, M.; Chen, B.; Duan, Y.; et al. Dual-Targeting to cancer cells and M2 macrophages via biomimetic delivery of mannosylated albumin nanoparticles for Drug-Resistant cancer therapy. Adv. Funct. Mater. 2017, 27, 1700403. [Google Scholar] [CrossRef]
- Chen, J.; Ouyang, J.; Chen, Q.; Deng, C.; Meng, F.; Zhang, J.; Cheng, R.; Lan, Q.; Zhong, Z. EGRF and CD44 Dual-Targeted multifunctional hyaluronic acid nanogels boost protein delivery to ovarian and breast cancers in vitro and in vivo. ACS Appl. Mater. Interfaces 2017, 9, 24140–24147. [Google Scholar] [CrossRef]
- Heo, R.; You, D.G.; Um, W.; Choi, K.Y.; Jeon, S.; Park, J.-S.; Choi, Y.; Kwon, S.; Kim, K.; Kwon, I.C.; et al. Dextran sulfate nanoparticles as a theranostic nanomedicine for rheumatoid arthritis. Biomaterials 2017, 131, 15–26. [Google Scholar] [CrossRef]
- Carradori, D.; Balducci, C.; Re, F.; Brambilla, D.; Le Droumaguet, B.; Flores, O.; Gaudin, A.; Mura, S.; Forloni, G.; Ordoñez-Gutierrez, L.; et al. Antibody-Functionalized polymer nanoparticle leading to memory recovery in Alzheimer’s Disease-Like transgenic mouse model. Nanomedicine 2018, 14, 609–618. [Google Scholar] [CrossRef]
- Li, N.; Sun, Q.; Yu, Z.; Gao, X.; Pan, W.; Wan, X.; Tang, B. Nuclear-Targeted photothermal therapy prevents cancer recurrence with near-infrared triggered copper sulfide nanoparticles. ACS Nano 2018, 12, 5197–5206. [Google Scholar] [CrossRef]
- Wang, H.; Yin, H.; Yan, F.; Sun, M.; Du, L.; Peng, W.; Li, Q.; Feng, Y.; Zhou, Y. Folate-Mediated mitochondrial targeting with Doxorubicin-Polyrotaxane nanoparticles overcomes multidrug resistance. Oncotarget 2015, 6, 2827–2842. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nag, O.K.; Delehanty, J.B. Active Cellular and Subcellular Targeting of Nanoparticles for Drug Delivery. Pharmaceutics 2019, 11, 543. https://doi.org/10.3390/pharmaceutics11100543
Nag OK, Delehanty JB. Active Cellular and Subcellular Targeting of Nanoparticles for Drug Delivery. Pharmaceutics. 2019; 11(10):543. https://doi.org/10.3390/pharmaceutics11100543
Chicago/Turabian StyleNag, Okhil K., and James B. Delehanty. 2019. "Active Cellular and Subcellular Targeting of Nanoparticles for Drug Delivery" Pharmaceutics 11, no. 10: 543. https://doi.org/10.3390/pharmaceutics11100543