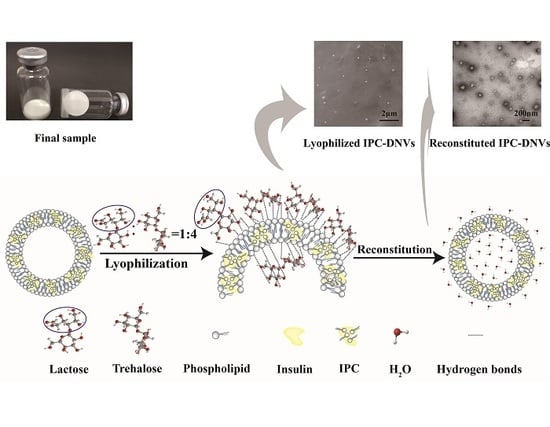
Stabilization of Deformable Nanovesicles Based on Insulin-Phospholipid Complex by Freeze-Drying
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Preparation of Stable Deformable Nanovesicles
2.3.1. Preparation of Nanovesicles
2.3.2. Lyophilization
2.3.3. Reconstitution
2.4. Determination of Particle Size, Polydispersity Index(PDI) and Density
2.5. Drug Content and Entrapment Efficiency (EE, %)
2.6. Deformability Index of Nanovesicles
2.7. X-Ray Diffraction (XRD)
2.8. Differential Scanning Calorimetry (DSC)
2.9. Microscopic Examination of Nanovesicles
2.9.1. Scanning Electron Microscopy (SEM)
2.9.2. Transmission Electron Microscopy (TEM)
2.10. Conformational Stability
2.11. Stability Study
2.12. Moisture Content Determination
2.13. In Vivo Study
2.13.1. Determination of Serum Insulin and Relative Insulin Bioavailability of Lyophilized IPC-DNVs
2.13.2. Bioactivity and In Vivo Hypoglycemic Effect of Lyophilized IPC-DNVs
2.14. Statistical Analysis
3. Results
3.1. Formulation Development and Corresponding Protection Mechanism Consideration
3.1.1. Effect of Different Density of Nanovesicles and Reconstituted Solvent
3.1.2. Effect of Single Cryoprotectant
3.1.3. Effect of Using Combinations of Cryoprotectants
3.1.4. Differential Scanning Calorimetry
3.1.5. Microscopic Examination of Nanovesicles
3.1.6. X-Ray Diffraction
3.1.7. Conformational Stability
3.1.8. Stability Studies
3.1.9. Moisture Content Determination
3.2. Bioavailability and In Vivo Hypoglycaemic Effect of Lyophilized IPC-DNVs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Elsayed, M.M.; Abdallah, O.Y.; Naggar, V.F.; Khalafallah, N.M. Lipid vesicles for skin delivery of drugs: reviewing three decades of research. Int. J. Pharm. 2007, 332, 1–16. [Google Scholar] [CrossRef]
- Cevc, G.; Blume, G. Lipid vesicles penetrate into intact skin owing to the transdermal osmotic gradients and hydration force. Biochim. Biophys. Acta 1992, 1104, 226–232. [Google Scholar] [CrossRef]
- Cevc, G. Transfersomes, liposomes and other lipid suspensions on the skin: permeation enhancement, vesicle penetration, and transdermal drug delivery. Ther. Drug Carrier Syst. 1996, 13, 257–388. [Google Scholar] [CrossRef]
- Touitou, E.; Dayan, N.; Bergelson, L.; Godin, B.; Eliaz, M. Ethosomes - novel vesicular carriers for enhanced delivery: characterization and skin penetration properties. J. Control. Release 2000, 65, 403–418. [Google Scholar] [CrossRef]
- Dragicevic-Curic, N.; Scheglmann, D.; Albrecht, V.; Fahr, A. Temoporfin-loaded invasomes: development, characterization and in vitro skin penetration studies. J. Control. Release 2008, 127, 59–69. [Google Scholar] [CrossRef]
- Kakkar, S.; Kaur, I.P. Spanlastics—A novel nanovesicular carrier system for ocular delivery. Int. J. Pharm. 2011, 413, 202–210. [Google Scholar] [CrossRef]
- Manca, M.L.; Zaru, M.; Manconi, M.; Lai, F.; Valenti, D.; Sinico, C.; Fadda, A. Glycerosomes: A new tool for effective dermal and transdermal drug delivery. Int. J. Pharm. 2013, 455, 66–74. [Google Scholar] [CrossRef]
- Mura, S.; Manconi, M.; Sinico, C.; Valenti, D.; Fadda, A.M. Penetration enhancer-containing vesicles (PEVs) as carriers for cutaneous delivery of minoxidil. Int. J. Pharm. 2009, 18, 1339–1345. [Google Scholar] [CrossRef] [PubMed]
- Manca, M.L.; Castangia, I.; Zaru, M.; Nácher, A.; Valenti, D.; Fernàndez-Busquets, X.; Fadda, A.M.; Manconi, M. Development of curcumin loaded sodium hyaluronate immobilized vesicles (hyalurosomes) and their potential on skin inflammation and wound restoring. Biomaterials 2015, 71, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Mai, R.; Gomaa, I.; Afifi, N.; Abdel-Kader, M. Dermal Delivery of Fe-Chlorophyllin via Ultradeformable Nanovesicles for Photodynamic Therapy in Melanoma Animal Model. Int. J. Pharm. 2018, 548, 480–490. [Google Scholar]
- Song, C.K.; Balakrishnan, P.; Shim, C.K.; Chung, S.J.; Chong, S.; Kim, D.D. A novel vesicular carrier, transethosome, for enhanced skin delivery of voriconazole: characterization and in vitro/in vivo evaluation. Colloid Surface B Biointerfaces 2012, 92, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Honeywell-Nguyen, P.L.; Bouwstra, J.A. Vesicles as a tool for transdermal and dermal delivery. Drug Discov. Today Technol. 2005, 2, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, N.; Andrade, F.; Segovia, N.; Ferrer-Tasies, L.; Sala, S.; Veciana, J.; Ventosa, N. Lipid-based nanovesicles for nanomedicine. Chem. Soc. Rev. 2016, 45, 6520–6545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Maghraby, G.M.M.; Williams, A.C.; Barry, B. Interactions of surfactants (edge activators) and skin penetration enhancers with liposomes. Int. J. Pharm. 2004, 276, 143–161. [Google Scholar] [CrossRef]
- Pathak, K.; Sharma, V.; Sharma, M. Optimization, in vitro cytotoxicity and penetration capability of deformable nanovesicles of paclitaxel for dermal chemotherapy in Kaposi sarcoma. Artificial Cells Nanomed. Biotechnol. 2015, 44, 1671–1683. [Google Scholar] [CrossRef] [PubMed]
- Grit, M.; Crommelin, D.J. Chemical stability of liposomes: implications for their physical stability. Chem. Phys. Lipids 1993, 64, 3–18. [Google Scholar] [CrossRef]
- Zuidam, N.J.; Gouw, H.K.M.E.; Barenholz, Y.; Crommelin, D.J.A. Physical (in) stability of liposomes upon chemical hydrolysis: the role of lysophospholipids and fatty acids. Biochim. Biophys. Acta 1995, 1240, 101–110. [Google Scholar] [CrossRef] [Green Version]
- Yusuf, M.; Sharma, V.; Pathak, K. Nanovesicles for transdermal delivery of felodipine: Development, characterization, and pharmacokinetics. J. Pharm. Investig. 2014, 4, 119–130. [Google Scholar] [Green Version]
- Maestrelli, F.; Capasso, G.; González-Rodríguez, M.L.; Rabasco, A.M.; Ghelardini, C.; Mura, P. Effect of preparation technique on the properties and in vivo efficacy of benzocaine-loaded ethosomes. J. Liposome Res. 2009, 19, 253–260. [Google Scholar] [CrossRef]
- Sala, M.; Locher, F.; Bonvallet, M.; Agusti, G.; Elaissari, A.; Fessi, H. Diclofenac Loaded Lipid Nanovesicles Prepared by Double Solvent Displacement for Skin Drug Delivery. Pharm. Res. 2017, 34, 1908–1924. [Google Scholar] [CrossRef]
- El Zaafarany, G.M.; Awad, G.A.; Holayel, S.M.; Mortada, N.D. Role of edge activators and surface charge in developing ultradeformable vesicles with enhanced skin delivery. Int. J. Pharm. 2010, 397, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Karthikeyan, C.; Trivedi, P. Localized delivery of cisplatin for the effective management of squamous cell carcinoma from protransfersome formulation. Arch. Pharm. Res. 2012, 35, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Dragicevic-Curic, N.; Susanna, G.; Gitter, B.; Winter, S.; Fahr, A. Surface charged temoporfin-loaded flexible vesicles: in vitro skin penetration studies and stability. Int. J. Pharm. 2010, 384, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Franzé, S.; Selmin, F.; Samaritani, E.; Minghetti, P.; Cilurzo, F. Lyophilization of liposomal formulations: still necessary, still challenging. Pharmaceutics 2018, 10, 139. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Suzuki, Y.; Kobayashi, Y.; Ishii, T.; Uchida, S.; Itaka, K.; Kataoka, K.; Okamoto, H. Development of biodegradable polycation-based inhalable dry gene powders by spray freeze drying. Pharmaceutics 2015, 7, 233–254. [Google Scholar] [CrossRef] [PubMed]
- Glavas-Dodov, M.; Fredro-Kumbaradzi, E.; Goracinova, K.; Simonoska, M.; Calis, S.; Trajkovic-Jolevska, T.; Hincal, A.A. The effects of lyophilization on the stability of liposomes containing 5-FU. Int. J. Pharm. 2005, 291, 79–86. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, F.M.; Yan, S.J. Preparation, in vitro release, and pharmacokinetics in rabbits of lyophilized injection of sorafenib solid lipid nanoparticles. Int. J. Nanomed. 2012, 7, 2901–2910. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Zhou, C.; Xia, X.; Liu, Y. Self-assembled lecithin/chitosan nanoparticles for oral insulin delivery: preparation and functional evaluation. Int. J. Nanomed. 2016, 11, 761–769. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, X.; Zhang, Y.; Ye, J.; Wang, H.L.; Xia, X.J.; Liu, Y. Mechanisms of deformable nanovesicles based on insulin-phospholipid complex for enhancing buccal delivery of insulin. Int. J. Nanomed. 2018, 13, 7319–7331. [Google Scholar] [CrossRef]
- Cevc, G. Chapter 9 Material transport across permeability barriers by means of lipid vesicles. Handb. Biol. Phys. 1995, 1, 465–490. [Google Scholar]
- Peer, D.; Florentin, A.; Margalit, R. Hyaluronan is a key component in cryoprotection and formulation of targeted unilamellar liposomes. Biochim. Biophys. Acta 2003, 1612, 76–82. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Han, D.; Cai, C.; Tang, X. An overview of liposome lyophilization and its future potential. J. Control. Release 2010, 142, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Pocker, Y.; Biswas, S.B. Conformational dynamics of insulin in solution. Circular dichroic studies. Biochemistry 1980, 19, 5043–5049. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Shields, J.E. pH dependent conformational changes in the T-and R-states of insulin in solution: circular dichroic studies in the pH range of 6 to 10. Biochem. Biophys. Res. Commun. 1992, 186, 1115–1120. [Google Scholar] [CrossRef]
- Mohammed, A.R.; Coombes, A.G.; Perrie, Y. Amino acids as cryoprotectants for liposomal delivery systems. Eur. J. Pharm. Sci. 2007, 30, 406–413. [Google Scholar] [CrossRef]
- Mulgund, S.V.; Phoujdar, M.S.; Londhe, S.V.; Mallade, P.S.; Kulkarni, T.S.; Deshpande, A.S.D.; Jain, K.S. Stability indicating HPLC method for simultaneous determination of mephenesin and diclofenac diethylamine. Indian J. Pharm. Sci. 2009, 71, 35–40. [Google Scholar] [Green Version]
- Chen, R.; Li, R.; Liu, Q.; Bai, C.; Qin, B.; Ma, Y.; Han, J. Ultradeformable liposomes: a novel vesicular carrier for enhanced transdermal delivery of procyanidins: effect of surfactants on the formation, stability, and transdermal delivery. AAPS PharmSciTech 2017, 18, 1823–1832. [Google Scholar] [CrossRef]
- Patist, A.; Zoerb, H. Preservation mechanisms of trehalose in food and biosystems. Colloids Surf. B Biointerfaces 2005, 40, 107–113. [Google Scholar] [CrossRef]
- Date, P.V.; Samad, A.; Devarajan, P.V. Freeze Thaw: A Simple Approach for Prediction of Optimal Cryoprotectant for Freeze Drying. AAPS Pharmscitech 2010, 11, 304–313. [Google Scholar] [CrossRef] [Green Version]
- Lerbret, A.; Bordat, P.; Affouard, F.; Guinet, Y.; Hédoux, A.; Paccou, L.; Prévost, D.; Descamps, M. Influence of homologous disaccharides on the hydrogen-bond network of water: complementary Raman scattering experiments and molecular dynamics simulations. Carbohydr. Res. 2005, 340, 881–887. [Google Scholar] [CrossRef]
- Ingvarsson, P.T.; Yang, M.; Nielsen, H.M.; Rantanen, J.; Foged, C. Stabilization of liposomes during drying. Expert Opinion on Drug Delivery. Expert Opin. Drug Del. 2011, 8, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Quintanarguerrero, D.; Ganemquintanar, A.; Allémann, E.; Fessi, H.; Doelker, E. Influence of the stabilizer coating layer on the purification and freeze-drying of poly(D,L-lactic acid) nanoparticles prepared by an emulsion-diffusion technique. J. Microencapsulation. 1998, 15, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Martins Sara, I.F.S.; Jongen, W.M.F.; Boekel, M.A.J.S.V. A review of Maillard reaction in food and implications to kinetic modelling. Trends Food Sci. Tech. 2000, 11, 364–373. [Google Scholar] [CrossRef]
- Jain, N.K.; Roy, I. Effect of trehalose on protein structure. Protein Sci. 2009, 18, 24–36. [Google Scholar] [CrossRef]










| Formulation | Density (per μm3) | Size (nm) | PDI | EE (%) | DI (µg/cm2/s) | |
|---|---|---|---|---|---|---|
| Dilution | 0 | 1000 | 149.87 ± 0.70 | 0.49 ± 0.00 | 80.92 ± 1.84 | 33.20 ± 0.21 |
| 1 | 500 | 109.23 ± 0.76 | 0.20 ± 0.09 | 80.50 ± 0.36 | 36.31 ± 2.44 | |
| 3 | 333 | 94.45 ± 3.11 | 0.28 ± 0.02 | 79.06 ± 0.20 | 37.13 ± 1.48 | |
| 5 | 200 | 85.37 ± 0.29 | 0.25 ± 0.01 | 60.69 ± 1.06 | 40.31 ± 2.81 | |
| Reconstituted Solvent | Size (nm) | PDI | EE (%) | DI (µg/cm2/s) |
|---|---|---|---|---|
| Phosphate buffer | 131.57 ± 1.62 | 0.21 ± 0.11 | 81.18 ± 0.52 | 31.04 ± 0.12 |
| Acetate buffer | 139.60 ± 0.56 | 0.26 ± 0.01 | 73.68 ± 0.49 | 33.92 ± 0.51 |
| Sodium chloride | 122.93 ± 0.61 | 0.21 ± 0.01 | 75.90 ± 0.49 | 36.51 ± 1.49 |
| Water | 105.57 ± 0.59 | 0.20 ± 0.00 | 83.37 ± 0.18 | 36.91 ± 1.41 |
| Cryoprot-ectant a | Reconstituted after Lyophilization | Storage at 25 °C for 1 month | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lyo b | Rec c | Size(nm) | PDI | EE (%) | DI (µg/cm2/s) | Lyo b | Rec c | Size(nm) | PDI | EE (%) | DI (µg/cm2/s) | |
| Glucose |  |  | 279.77 ± 2.31 | 0.45 ± 0.00 | 73.16 ± 1.91 | 23.09 ± 3.54 |  |  | 335.10 ± 3.62 | 0.62 ± 0.13 | 67.42 ± 2.11 | 22.15 ± 3.79 |
| Sucrose |  |  | 181.83 ± 0.25 | 0.28 ± 0.01 | 71.19 ± 5.26 | 32.02 ± 4.29 |  |  | 237.30 ± 1.15 | 0.34 ± 0.00 | 68.86 ± 2.74 | 27.91 ± 3.46 |
| Lactose |  |  | 151.03 ± 0.46 | 0.16 ± 0.01 | 70.19 ± 2.13 | 34.03 ± 3.72 |  |  | 155.07 ± 0.31 | 0.16 ± 0.01 | 70.02 ± 0.69 | 34.21 ± 4.80 |
| Trehalose |  |  | 105.57 ± 0.59 | 0.22 ± 0.00 | 80.68 ± 1.05 | 37.62 ± 3.18 |  |  | 128.23 ± 2.95 | 0.32 ± 0.01 | 84.62 ± 0.18 | 34.29 ± 2.52 |
| Mannitol |  |  | 450.70 ± 5.99 | 0.37 ± 0.13 | 64.31 ± 9.88 | 22.05 ± 1.39 |  |  | 627.97 ± 5.62 | 0.43 ± 0.03 | 24.45 ± 9.17 | 21.01 ± 1.86 |
| Inulin |  |  | 385.53 ± 9.32 | 0.31 ± 0.19 | 73.53 ± 4.29 | 14.59 ± 4.50 |  |  | 409.44 ± 1.49 | 0.31 ± 0.08 | 73.01 ± 3.24 | 10.39 ± 2.85 |
| Glycine |  |  | 249.28 ± 6.20 | 0.40 ± 0.12 | 64.20 ± 3.19 | 29.94 ± 2.05 |  |  | 269.77 ± 3.40 | 0.41 ± 0.06 | 63.05 ± 5.12 | 27.45 ± 3.07 |
| Hyaluronan |  |  | 835.87 ± 50.00 | 0.60 ± 0.09 | 80.32 ± 1.07 | 8.39 ± 1.94 |  |  | - | - | 82.33 ± 0.46 | 3.21 ± 0.30 |
 non-caked, critically shrunken, sprayed, or loose,
non-caked, critically shrunken, sprayed, or loose,  slightly shrunken,
slightly shrunken,  caked and compact, c: reconstituted appearance,
caked and compact, c: reconstituted appearance,  aggregated,
aggregated,  translucent. “-” means the data could not be obtained because the reconstituted samples were aggregated.
translucent. “-” means the data could not be obtained because the reconstituted samples were aggregated.| Ratio (w/w) | Size (nm) | PDI | EE (%) | DI (µg/cm2/s) | Size (nm) | PDI | EE (%) | DI (µg/cm2/s) | |
|---|---|---|---|---|---|---|---|---|---|
| Reconstituted after Lyophilization | |||||||||
| 1:2 | 131.67 ± 0.42 | 0.20 ± 0.02 | 73.76 ± 0.42 | 35.42 ± 2.52 | 3% | 149.27 ± 2.01 | 0.49 ± 0.00 | 59.26 ± 2.49 | 37.21 ± 3.07 |
| 1:4 | 109.47 ± 0.49 | 0.18 ± 0.01 | 80.80 ± 1.48 | 38.47 ± 4.13 | 5% | 122.33 ± 1.03 | 0.28 ± 0.01 | 77.47 ± 1.88 | 37.91 ± 4.18 |
| 1:6 | 111.00 ± 0.72 | 0.22 ± 0.01 | 80.82 ± 0.97 | 37.95 ± 3.45 | 8% | 109.47 ± 0.49 | 0.18 ± 0.01 | 80.80 ± 1.48 | 38.47 ± 4.13 |
| 1:8 | 111.60 ± 0.46 | 0.26 ± 0.00 | 80.79 ± 0.78 | 37.85 ± 2.57 | 10% | 150.53 ± 1.66 | 0.45 ± 0.01 | 80.68 ± 1.05 | 32.94 ± 1.09 |
| 13% | 175.00 ± 1.13 | 0.28 ± 0.22 | 83.77 ± 1.60 | 32.00 ± 2.49 | |||||
| Storage at 25°C for 1 month | |||||||||
| 1:2 | 149.00 ± 0.99 | 0.48 ± 0.01 | 71.47 ± 1.23 | 32.95 ± 3.25 | 3% | 170.80 ± 0.46 | 0.43 ± 0.01 | 51.71 ± 3.45 | 28.39 ± 4.80 |
| 1:4 | 110.10 ± 0.78 | 0.17 ± 0.01 | 82.24 ± 3.65 | 37.59 ± 2.02 | 5% | 130.57 ± 0.80 | 0.29 ± 0.12 | 83.12 ± 1.68 | 37.48 ± 3.32 |
| 1:6 | 114.80 ± 0.30 | 0.29 ± 0.02 | 80.64 ± 2.29 | 35.73 ± 3.08 | 8% | 110.10 ± 0.78 | 0.22 ± 0.01 | 82.24 ± 0.36 | 37.59 ± 2.02 |
| 1:8 | 123.17 ± 0.45 | 0.27 ± 0.00 | 78.14 ± 0.81 | 33.48 ± 4.19 | 10% | 159.00 ± 0.27 | 0.16 ± 0.02 | 81.73 ± 1.31 | 32.52 ± 2.91 |
| 13% | 174.43 ± 2.29 | 0.27 ± 0.01 | 80.74 ± 2.12 | 30.31 ± 1.14 | |||||
| Storage at 25°C for 2 months | |||||||||
| 1:2 | 156.17 ± 0.61 | 0.15 ± 0.01 | 70.84 ± 2.70 | 25.01 ± 2.75 | 3% | 198.70 ± 2.00 | 0.45 ± 0.02 | 41.26 ± 5.80 | 20.52 ± 2.45 |
| 1:4 | 119.77 ± 0.45 | 0.18 ± 0.02 | 81.50 ± 7.97 | 37.02 ± 4.50 | 5% | 141.10 ± 1.22 | 0.33 ± 0.07 | 80.28 ± 5.18 | 38.26 ± 2.71 |
| 1:6 | 119.37 ± 0.25 | 0.33 ± 0.01 | 80.25 ± 2.63 | 34.42 ± 4.50 | 8% | 119.77 ± 0.45 | 0.21 ± 0.01 | 81.50 ± 7.97 | 37.02 ± 4.50 |
| 1:8 | 176.70 ± 0.72 | 0.58 ± 0.02 | 74.32 ± 2.77 | 29.84 ± 4.45 | 10% | 168.47 ± 0.55 | 0.43 ± 0.01 | 84.55 ± 2.17 | 28.75 ± 3.41 |
| 13% | 181.8 ± 1.14 | 0.47 ± 0.01 | 85.20 ± 1.57 | 30.16 ± 2.95 | |||||
| Formulation | Tg values(°C) |
|---|---|
| 8%-T | 57.12 |
| 8%-L | 38.09 |
| 8%-L-T | 50.42 |
| Formulation | α-helix Ratio (%) | θ208/θ223 Ratio |
|---|---|---|
| Insulin | 23.5 | 1.62 |
| IPC-DNVs | 23.3 | 1.63 |
| 8%-L | 35.9 | 2.30 |
| 8%-T | 24.1 | 1.42 |
| 8%-L-T | 23.8 | 1.61 |
| Formulation | AUC0→6h (mU/L·h) | Fr (%) | AAC0→6h (%·h) | Fp (%) |
|---|---|---|---|---|
| Insulin solution (1 IU/kg, SC) | 239.45 ± 45.08 | / | 163.42 ± 30.21 | / |
| IPC-DNVs (10 IU/kg, buccally) | 437.44 ± 68.55* | 18.27 | 247.22 ± 4.82** | 15.74 |
| 8%-L-T (10 IU/kg, buccally) | 418.00 ± 51.45* | 17.45 | 236.61 ± 37.94* | 14.49 |
| 8%-L-T-SC (1 IU/kg, SC) | 235.95 ± 54.49 | 98.54 | 157.89 ± 15.07 | 96.61 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Guo, Y.; Yang, Y.; Meng, Y.; Xia, X.; Liu, Y. Stabilization of Deformable Nanovesicles Based on Insulin-Phospholipid Complex by Freeze-Drying. Pharmaceutics 2019, 11, 539. https://doi.org/10.3390/pharmaceutics11100539
Xu Y, Guo Y, Yang Y, Meng Y, Xia X, Liu Y. Stabilization of Deformable Nanovesicles Based on Insulin-Phospholipid Complex by Freeze-Drying. Pharmaceutics. 2019; 11(10):539. https://doi.org/10.3390/pharmaceutics11100539
Chicago/Turabian StyleXu, You, Yiyue Guo, Yuqi Yang, Yingying Meng, Xuejun Xia, and Yuling Liu. 2019. "Stabilization of Deformable Nanovesicles Based on Insulin-Phospholipid Complex by Freeze-Drying" Pharmaceutics 11, no. 10: 539. https://doi.org/10.3390/pharmaceutics11100539