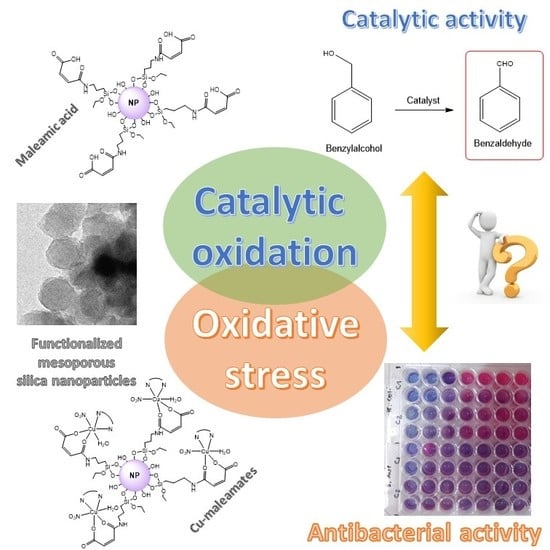
Preparation and Study of the Antibacterial Applications and Oxidative Stress Induction of Copper Maleamate-Functionalized Mesoporous Silica Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Remarks on the Synthesis of Materials
2.2. General Remarks on the Characterization of Materials
2.3. Synthesis of Mesoporous Silica Nanoparticles (MSN Material)
2.4. Preparation of MSN-Maleamic
2.5. Preparation of MSN-Maleamic-Cu
2.6. Antibacterial Studies
2.7. Oxidative Stress Studies
2.8. Catalytic Oxidation of Benzyl alcohol
3. Results and Discussion
3.1. Synthesis and Characterization of Maleamic-Functionalized Mesoporous Silica Nanoparticles
3.1.1. Quantification of the Functionalization Rate by Thermogravimetry and X-ray Fluorescence
3.1.2. Characterization by Powder X-ray Diffraction Studies
3.1.3. Nitrogen Adsorption–Desorption Isotherms
3.1.4. Solid-State NMR Spectroscopy
3.1.5. DR-UV and FT-IR Studies
3.1.6. TEM Studies
3.2. Biological Studies of the Synthesized Materials
3.2.1. Antibacterial Tests
3.2.2. Oxidative Stress Studies
3.3. Study of the Catalytic Oxidation Activity of the Synthesized Materials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Manzano, M.; Vallet-Regí, M. Mesoporous silica nanoparticles in nanomedicine applications. J. Mater. Sci. Mater. Med. 2018, 29, 65. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Sen, K. Contemporary mesoporous materials for drug delivery applications: A review. J. Porous Mater. 2018, 25, 965–987. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Colilla, M.; Izquierdo-Barba, I.; Manzano, M.; Vallet-Regí, M.; Colilla, M.; Izquierdo-Barba, I.; Manzano, M. Mesoporous Silica Nanoparticles for Drug Delivery: Current Insights. Molecules 2017, 23, 47. [Google Scholar] [CrossRef] [PubMed]
- Bollu, V.S.; Barui, A.K.; Mondal, S.K.; Prashar, S.; Fajardo, M.; Briones, D.; Rodríguez-Diéguez, A.; Patra, C.R.; Gómez-Ruiz, S. Curcumin-loaded silica-based mesoporous materials: Synthesis, characterization and cytotoxic properties against cancer cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 63, 393–410. [Google Scholar] [CrossRef] [PubMed]
- Kotcherlakota, R.; Barui, A.K.; Prashar, S.; Fajardo, M.; Briones, D.; Rodríguez-Diéguez, A.; Patra, C.R.; Gómez-Ruiz, S. Curcumin loaded mesoporous silica: An effective drug delivery system for cancer treatment. Biomater. Sci. 2016, 4, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Teng, B.; Han, Y.; Zhang, X.; Xiao, H.; Yu, C.; Li, H.; Cheng, Z.; Jin, D.; Wong, K.-L.; Ma, P.; et al. Phenanthriplatin(IV) conjugated multifunctional up-converting nanoparticles for drug delivery and biomedical imaging. J. Mater. Chem. B 2018, 6, 5059–5068. [Google Scholar] [CrossRef]
- Pasha, S.S.; Fageria, L.; Climent, C.; Rath, N.P.; Alemany, P.; Chowdhury, R.; Roy, A.; Laskar, I.R. Evaluation of novel platinum(II) based AIE compound-encapsulated mesoporous silica nanoparticles for cancer theranostic application. Dalton Trans. 2018, 47, 4613–4624. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, P.; He, Z.; He, H.; Rong, W.; Li, J.; Zhou, D.; Huang, Y. Mesoporous silica nanoparticles with lactose-mediated targeting effect to deliver platinum(IV) prodrug for liver cancer therapy. J. Mater. Chem. B 2017, 5, 7591–7597. [Google Scholar] [CrossRef]
- Del Hierro, I.; Pérez, Y.; Cruz, P.; Juárez, R. Pt and Ti Complexes Immobilized onto Mesoporous Silica Microspheres and Their Interaction with Molecules of Biological Interest. Eur. J. Inorg. Chem. 2017, 3030–3039. [Google Scholar] [CrossRef]
- Zheng, Y.; Fahrenholtz, C.D.; Hackett, C.L.; Ding, S.; Day, C.S.; Dhall, R.; Marrs, G.S.; Gross, M.D.; Singh, R.; Bierbach, U. Large-Pore Functionalized Mesoporous Silica Nanoparticles as Drug Delivery Vector for a Highly Cytotoxic Hybrid Platinum–Acridine Anticancer Agent. Chem. Eur. J. 2017, 23, 3386–3397. [Google Scholar] [CrossRef] [PubMed]
- Edeler, D.; Kaluderovic, M.R.; Dojcinovic, B.; Schmidt, H.; Kaluderovic, G.N. SBA-15 mesoporous silica particles loaded with cisplatin induce senescence in B16F10 cells. RSC Adv. 2016, 6, 111031–111040. [Google Scholar] [CrossRef]
- Ravera, M.; Gabano, E.; Zanellato, I.; Perin, E.; Arrais, A.; Osella, D. Functionalized nonporous silica nanoparticles as carriers for Pt(IV) anticancer prodrugs. Dalton Trans. 2016, 45, 17233–17240. [Google Scholar] [CrossRef] [PubMed]
- Wani, W.A.; Prashar, S.; Shreaz, S.; Gómez-Ruiz, S. Nanostructured materials functionalized with metal complexes: In search of alternatives for administering anticancer metallodrugs. Coord. Chem. Rev. 2016, 312, 67–98. [Google Scholar] [CrossRef]
- Pérez-Quintanilla, D.; Gómez-Ruiz, S.; Žižak, Ž.; Sierra, I.; Prashar, S.; del Hierro, I.; Fajardo, M.; Juranić, Z.D.; Kaluđerović, G.N. A New Generation of Anticancer Drugs: Mesoporous Materials Modified with Titanocene Complexes. Chem. Eur. J. 2009, 15, 5588–5597. [Google Scholar] [CrossRef] [PubMed]
- Kaluderović, G.N.; Pérez-Quintanilla, D.; Zizak, Z.; Juranić, Z.D.; Gómez-Ruiz, S. Improvement of cytotoxicity of titanocene-functionalized mesoporous materials by the increase of the titanium content. Dalton Trans. 2010, 39, 2597–2608. [Google Scholar] [CrossRef] [PubMed]
- Kaluđerović, G.N.; Pérez-Quintanilla, D.; Sierra, I.; Prashar, S.; del Hierro, I.; Žižak, Ž.; Juranić, Z.D.; Fajardo, M.; Gómez-Ruiz, S. Study of the influence of the metal complex on the cytotoxic activity of titanocene-functionalized mesoporous materials. J. Mater. Chem. 2010, 20, 806–814. [Google Scholar] [CrossRef]
- García-Peñas, A.; Gómez-Ruiz, S.; Pérez-Quintanilla, D.; Paschke, R.; Sierra, I.; Prashar, S.; del Hierro, I.; Kaluđerović, G.N. Study of the cytotoxicity and particle action in human cancer cells of titanocene-functionalized materials with potential application against tumors. J. Inorg. Biochem. 2012, 106, 100–110. [Google Scholar] [CrossRef]
- Ceballos-Torres, J.; Virag, P.; Cenariu, M.; Prashar, S.; Fajardo, M.; Fischer-Fodor, E.; Gómez-Ruiz, S. Anti-cancer Applications of Titanocene-Functionalised Nanostructured Systems: An Insight into Cell Death Mechanisms. Chem. Eur. J. 2014, 20, 10811–10828. [Google Scholar] [CrossRef]
- Ceballos-Torres, J.; Prashar, S.; Fajardo, M.; Chicca, A.; Gertsch, J.; Pinar, A.B.; Gómez-Ruiz, S. Ether-Substituted Group 4 Metallocene Complexes: Cytostatic Effects and Applications in Ethylene Polymerization. Organometallics 2015, 34, 2522–2532. [Google Scholar] [CrossRef]
- Gómez-Ruiz, S.; García-Peñas, A.; Prashar, S.; Rodríguez-Diéguez, A.; Fischer-Fodor, E. Anticancer Applications of Nanostructured Silica-Based Materials Functionalized with Titanocene Derivatives: Induction of Cell Death Mechanism through TNFR1 Modulation. Materials 2018, 11, 224. [Google Scholar] [CrossRef] [PubMed]
- Díaz-García, D.; Cenariu, D.; Pérez, Y.; Cruz, P.; del Hierro, I.; Prashar, S.; Fischer-Fodor, E.; Gómez-Ruiz, S. Modulation of the mechanism of apoptosis in cancer cell lines by treatment with silica-based nanostructured materials functionalized with different metallodrugs. Dalton Trans. 2018, 47, 12284–12299. [Google Scholar] [CrossRef] [PubMed]
- Del Hierro, I.; Gómez-Ruiz, S.; Pérez, Y.; Cruz, P.; Prashar, S.; Fajardo, M. Mesoporous SBA-15 modified with titanocene complexes and ionic liquids: Interactions with DNA and other molecules of biological interest studied by solid state electrochemical techniques. Dalton Trans. 2018, 47, 12914–12932. [Google Scholar] [CrossRef] [PubMed]
- Bulatović, M.Z.; Maksimović-Ivanić, D.; Bensing, C.; Gómez-Ruiz, S.; Steinborn, D.; Schmidt, H.; Mojić, M.; Korać, A.; Golić, I.; Pérez-Quintanilla, D.; et al. Organotin(IV)-loaded mesoporous silica as a biocompatible strategy in cancer treatment. Angew. Chem. Int. Ed. Engl. 2014, 53, 5982–5987. [Google Scholar] [CrossRef]
- Bensing, C.; Mojić, M.; Gómez-Ruiz, S.; Carralero, S.; Dojčinović, B.; Maksimović-Ivanić, D.; Mijatović, S.; Kaluđerović, G.N. Evaluation of functionalized mesoporous silica SBA-15 as a carrier system for Ph3Sn(CH2)3OH against the A2780 ovarian carcinoma cell line. Dalton Trans. 2016, 45, 18984–18993. [Google Scholar] [CrossRef]
- Ellahioui, Y.; Patra, M.; Mari, C.; Kaabi, R.; Karges, J.; Gasser, G.; Gómez-Ruiz, S. Mesoporous silica nanoparticles functionalised with a photoactive ruthenium(II) complex: Exploring the formulation of a metal-based photodynamic therapy photosensitiser. Dalton Trans. 2019. [Google Scholar] [CrossRef]
- González, B.; Colilla, M.; Díez, J.; Pedraza, D.; Guembe, M.; Izquierdo-Barba, I.; Vallet-Regí, M. Mesoporous silica nanoparticles decorated with polycationic dendrimers for infection treatment. Acta Biomater. 2018, 68, 261–271. [Google Scholar] [CrossRef]
- Michailidis, M.; Sorzabal-Bellido, I.; Adamidou, E.A.; Diaz-Fernandez, Y.A.; Aveyard, J.; Wengier, R.; Grigoriev, D.; Raval, R.; Benayahu, Y.; D’Sa, R.A.; et al. Modified Mesoporous Silica Nanoparticles with a Dual Synergetic Antibacterial Effect. ACS Appl. Mater. Interfaces 2017, 9, 38364–38372. [Google Scholar] [CrossRef]
- Tian, Y.; Qi, J.; Zhang, W.; Cai, Q.; Jiang, X. Facile, One-Pot Synthesis, and Antibacterial Activity of Mesoporous Silica Nanoparticles Decorated with Well-Dispersed Silver Nanoparticles. ACS Appl. Mater. Interfaces 2014, 6, 12038–12045. [Google Scholar] [CrossRef]
- Tahmasbi, L.; Sedaghat, T.; Motamedi, H.; Kooti, M. Mesoporous silica nanoparticles supported copper(II) and nickel(II) Schiff base complexes: Synthesis, characterization, antibacterial activity and enzyme immobilization. J. Solid State Chem. 2018, 258, 517–525. [Google Scholar] [CrossRef]
- Kincaid, V.A.; Sullivan, E.D.; Klein, R.D.; Noel, J.W.; Rowlett, R.S.; Snider, M.J. Structure and Catalytic Mechanism of Nicotinate (Vitamin B3) Degradative Enzyme Maleamate Amidohydrolase from Bordetella bronchiseptica RB50. Biochemistry 2012, 51, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Burdon, R.C.F.; Junker, R.R.; Scofield, D.G.; Parachnowitsch, A.L. Bacteria colonising Penstemon digitalis show volatile and tissue-specific responses to a natural concentration range of the floral volatile linalool. Chemoecology 2018, 28, 11–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamrani, O.; Boutamine, S.; Kellou-Tairi, S.; Hank, Z. Copper-Drug Based Complexes: Antimicrobial, Antioxidant and Pharmacological Study. Glob. J. Nano 2017, 3, 555610. [Google Scholar]
- Katugampala, S.; Perera, I.C.; Nanayakkara, C.; Perera, T. Synthesis, Characterization, and Antimicrobial Activity of Novel Sulfonated Copper-Triazine Complexes. Bioinorg. Chem. Appl. 2018, 2018, 2530851. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.Y.; Basyouni, W.M.; El-Bayouki, K.A.M. Synthesis, characterization and antimicrobial activity of 5-(arylazo)salicylaldimines and their copper(II) complexes. Appl. Organomet. Chem. 2018, 32, e4032. [Google Scholar] [CrossRef]
- Savithri, K.; Kumar, B.C.V.; Vivek, H.K.; Revanasiddappa, H.D. Synthesis and Characterization of Cobalt(III) and Copper(II) Complexes of 2-((E)-(6-Fluorobenzo[d]thiazol-2-ylimino) methyl)-4-chlorophenol: DNA Binding and Nuclease Studies—Sod and Antimicrobial Activities. Int. J. Spectrosc. 2018, 2018, 8759372. [Google Scholar] [CrossRef]
- Brahma, U.; Kothari, R.; Sharma, P.; Bhandari, V. Antimicrobial and anti-biofilm activity of hexadentated macrocyclic complex of copper(II) derived from thiosemicarbazide against Staphylococcus aureus. Sci. Rep. 2018, 8, 8050. [Google Scholar] [CrossRef]
- Nomiya, K.; Morozumi, S.; Yanagawa, Y.; Hasegawa, M.; Kurose, K.; Taguchi, K.; Sakamoto, R.; Mihara, K.; Kasuga, N.C. Syntheses, Structures, and Antimicrobial Activities of Gold(I)- and Copper(I)-N-Heterocyclic Carbene (NHC) Complexes Derived from Basket-Shaped Dinuclear Ag(I)–NHC Complex. Inorg. Chem. 2018, 57, 11322–11332. [Google Scholar] [CrossRef]
- Lazarou, K.N.; Chadjistamatis, I.; Psycharis, V.; Perlepes, S.P.; Raptopoulou, C.P. First use of the maleamate(-1) ligand in coordination chemistry: Dinuclear copper(II) complexes with N-donors and their interesting “organic” chemistry. Inorg. Chem. Commun. 2007, 10, 318–323. [Google Scholar] [CrossRef]
- Lazarou, K.N.; Perlepes, S.P.; Psycharis, V.; Raptopoulou, C.P. Synthetic study of the ternary copper(II)/maleamate(-1)/1,10-phenanthroline reaction system: Mononuclear, dinuclear and polymeric complexes. Polyhedron 2008, 27, 2131–2142. [Google Scholar] [CrossRef]
- Lazarou, K.N.; Psycharis, V.; Perlepes, S.P.; Raptopoulou, C.P. Complexes derived from the copper(II) perchlorate/maleamic acid/2,2′-bipyridine and copper(II) perchlorate/maleic acid/2,2′-bipyridine reaction systems: Synthetic, reactivity, structural and spectroscopic studies. Polyhedron 2009, 28, 1085–1096. [Google Scholar] [CrossRef]
- Lazarou, K.N.; Boudalis, A.K.; Perlepes, S.P.; Terzis, A.; Raptopoulou, C.P. Maleamate(-1) and Maleate(-2) Copper(II)-2,2′-Bipyridine Complexes: Synthesis, Reactivity and Structural and Physical Studies. Eur. J. Inorg. Chem. 2009, 2009, 4554–4563. [Google Scholar] [CrossRef]
- Lazarou, K.N.; Raptopoulou, C.P.; Perlepes, S.P.; Psycharis, V. Complexes derived from the general copper(II)/maleamic acid/N,N′,N′′-chelate reaction systems: Synthetic, reactivity, structural and spectroscopic studies. Polyhedron 2009, 28, 3185–3192. [Google Scholar] [CrossRef]
- Yu, Z.; Cowan, J.A. Catalytic Metallodrugs: Substrate-Selective Metal Catalysts as Therapeutics. Chem. Eur. J. 2017, 23, 14113–14127. [Google Scholar] [CrossRef] [PubMed]
- Soldevila-Barreda, J.J.; Sadler, P.J. Approaches to the design of catalytic metallodrugs. Curr. Opin. Chem. Biol. 2015, 25, 172–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomás-Gamasa, M.; Martínez-Calvo, M.; Couceiro, J.R.; Mascareñas, J.L. Transition metal catalysis in the mitochondria of living cells. Nat. Commun. 2016, 7, 12538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubio-Ruiz, B.; Weiss, J.T.; Unciti-Broceta, A. Efficient Palladium-Triggered Release of Vorinostat from a Bioorthogonal Precursor. J. Med. Chem. 2016, 59, 9974–9980. [Google Scholar] [CrossRef] [PubMed]
- Balbín, A.; Gaballo, F.; Ceballos-Torres, J.; Prashar, S.; Fajardo, M.; Kaluđerović, G.N.; Gómez-Ruiz, S. Dual application of Pd nanoparticles supported on mesoporous silica SBA-15 and MSU-2: Supported catalysts for C–C coupling reactions and cytotoxic agents against human cancer cell lines. RSC Adv. 2014, 4, 54775–54787. [Google Scholar] [CrossRef]
- Liu, S.; Chen, H.; Deng, C.; Zhang, X.; Yang, P. Facile Synthesis of Copper(II)Immobilized on Magnetic Mesoporous Silica Microspheres for Selective Enrichment of Peptides for Mass Spectrometry Analysis. Angew. Chem. Int. Ed. 2010, 49, 7557–7561. [Google Scholar] [CrossRef]
- Tsiourvas, D.; Papavasiliou, A.; Deze, E.G.; Papageorgiou, S.K.; Katsaros, F.K.; Romanos, G.E.; Poulakis, E.; Philippopoulos, C.J.; Xin, Q.; Cool, P. A Green Route to Copper Loaded Silica Nanoparticles Using Hyperbranched Poly(Ethylene Imine) as Biomimetic Template: Application in Heterogeneous Catalysis. Catalysts 2017, 7, 390. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Felpin, F.-X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-Based Nanoparticles: Synthesis and Applications in Catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef] [PubMed]
- Kankala, R.K.; Liu, C.-G.; Chen, A.-Z.; Wang, S.-B.; Xu, P.-Y.; Mende, L.K.; Liu, C.-L.; Lee, C.-H.; Hu, Y.-F. Overcoming Multidrug Resistance through the Synergistic Effects of Hierarchical pH-Sensitive, ROS-Generating Nanoreactors. ACS Biomater. Sci. Eng. 2017, 3, 2431–2442. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard, M07-A8; CLSI: Wayne, PA, USA, 2011. [Google Scholar]
- Zhao, Y.; Trewyn, B.G.; Slowing, I.I.; Lin, V.S.-Y. Mesoporous Silica Nanoparticle-Based Double Drug Delivery System for Glucose-Responsive Controlled Release of Insulin and Cyclic AMP. J. Am. Chem. Soc. 2009, 131, 8398–8400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodríguez-Reinoso, F.; Rouquerol, J.; Thommes, M. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Wolińska, E.; Karczmarzyk, Z.; Wysocki, W. Structural characterization of copper complexes with chiral 1,2,4-triazine-oxazoline ligands. Heterocycl. Commun. 2016, 22, 265–274. [Google Scholar] [CrossRef]
- Mallick, S.; Sharma, S.; Banerjee, M.; Ghosh, S.S.; Chattopadhyay, A.; Paul, A. Iodine-Stabilized Cu Nanoparticle Chitosan Composite for Antibacterial Applications. ACS Appl. Mater. Interfaces 2012, 4, 1313–1323. [Google Scholar] [CrossRef] [PubMed]
- Aazam, E.S.; El-Said, W.A. Synthesis of copper/nickel nanoparticles using newly synthesized Schiff-base metals complexes and their cytotoxicity/catalytic activities. Bioorg. Chem. 2014, 57, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Cai, X.; Shi, Q.S.; Liu, L.; Wan, D.; Tan, S.; Ouyang, Y. Poly-l-lysine-modified reduced graphene oxide stabilizes the copper nanoparticles with higher water-solubility and long-term additively antibacterial activity. Colloids Surf. B Biointerfaces 2013, 107, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Gao, Y.; Zhang, H.; Feng, X.; Cai, H.; Liu, Y. Preparation and characterization of core–shell structure of SiO2@Cu antibacterial agent. Colloids Surf. B Biointerfaces 2010, 81, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Matynia, B.; Młodzinska, E.; Hryniewicz, W. Antimicrobial susceptibility patterns of Staphylococcus aureus in Poland obtained by the National Quality Assurance Programme. Clin. Microbiol. Infect. 2005, 11, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, E.; Zarazaga, M.; Sáenz, Y.; Briñas, L.; Torres, C. Mechanisms of Antibiotic Resistance in Escherichia coli Isolates Obtained from Healthy Children in Spain. Microb. Drug Resist. 2002, 8, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Albesa, I.; Becerra, M.C.; Battán, P.C.; Páez, P.L. Oxidative stress involved in the antibacterial action of different antibiotics. Biochem. Biophys. Res. Commun. 2004, 317, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Durán, N.; Durán, M.; de Jesus, M.B.; Seabra, A.B.; Fávaro, W.J.; Nakazato, G. Silver nanoparticles: A new view on mechanistic aspects on antimicrobial activity. Nanomedicine 2016, 12, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Lehman, S.E.; Morris, A.S.; Mueller, P.S.; Salem, A.K.; Grassian, V.H.; Larsen, S.C. Silica Nanoparticle-Generated ROS as a Predictor of Cellular Toxicity: Mechanistic Insights and Safety by Design. Environ. Sci. Nano 2015, 3, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Zhang, S.; Gu, F.; Ping, Y.; Guo, X.; Zhong, Z.; Su, F. Highly selective gas-phase oxidation of benzyl alcohol to benzaldehyde over silver-containing hexagonal mesoporous silica. Microporous Mesoporous Mater. 2012, 149, 158–165. [Google Scholar] [CrossRef]
- Cruz, P.; Pérez, Y.; del Hierro, I.; Fajardo, M. Copper, copper oxide nanoparticles and copper complexes supported on mesoporous SBA-15 as catalysts in the selective oxidation of benzyl alcohol in aqueous phase. Microporous Mesoporous Mater. 2016, 220, 136–147. [Google Scholar] [CrossRef]
- Yuan, W.; Zhang, C.; Wei, H.; Wang, W.; Li, K. In situ synthesis and immobilization of a Cu(II)–pyridyl complex on silica microspheres as a novel Fenton-like catalyst for RhB degradation at nearneutral pH. RSC Adv. 2017, 7, 22825–22835. [Google Scholar] [CrossRef]
- Lee, H.; Lee, H.-J.; Sedlak, D.L.; Lee, C. pH-Dependent reactivity of oxidants formed by iron and copper-catalyzed decomposition of hydrogen peroxide. Chemosphere 2013, 92, 652–658. [Google Scholar] [CrossRef]










| Material | Theoretical Cu wt % | Experimental Cu wt % 2 | Mass Loss % 1 | mmol of Maleamic or Cu/g Material |
|---|---|---|---|---|
| MSN-maleamic | - | - | 46.3 | 1.45 (maleamic) 1 |
| MSN-maleamic-Cu | 10 | 3.24 | 42.1 | 0.51 (Cu) 2 |
| Material | hkl | 2θ (°) | dhkl (nm) | a0 (nm) |
|---|---|---|---|---|
| MSN-maleamic | 100 | 2.15 | 4.099 | 4.733 |
| 110 | 3.71 | - | - | |
| 200 | 4.28 | - | - | |
| MSN-maleamic | 100 | 2.18 | 4.053 | 4.680 |
| MSN-maleamic-Cu | 100 | 2.18 | 4.023 | 4.645 |
| Material | SBET (m2/g) | Total Pore Volume (cm3/g) | dp (BJH) 1 (nm) |
|---|---|---|---|
| MSN | 853.03 | 0.73 | 3.42 |
| MSN-maleamic-Cu | 414.80 | 0.32 | 3.11 |
| Material | Staphylococcus Aureus ATCC 29213 | Escherichia Coli ATCC 25922 | ||
|---|---|---|---|---|
| MIC (µg/mL) | MBC (µg/mL) | MIC (µg/mL) | MBC (µg/mL) | |
| MSN-maleamic | 250 [41.7] | >250 [>41.7] | 62.5 [10.4] | 125 [20.9] |
| MSN-maleamic-Cu | >250 [>8.1] | >250 [>8.1] | 125 [4.1] | 125 [4.1] |
| Material | Benzaldehyde | Benzyl Benzoate | Time (h) | Total TON | ||||
|---|---|---|---|---|---|---|---|---|
| TON | TOF (h−1) | Selectivity (%) | TON | TOF (h−1) | Selectivity (%) | |||
| MSN | 0.34 ± 0.28 | 0.014 ± 0.011 | 83.7 ± 16.1 | 0.11 ± 0.04 | 0.005 ± 0.002 | 16.3 ± 16.1 | 24 | 0.45 ± 0.32 |
| MSN-maleamic | 0.69 ± 0.24 | 0.116 ± 0.041 | 92.1 ± 1.9 | 0.06 ± 0.02 | 0.002 ± 0.001 | 7.9 ± 1.9 | 6 | 0.75 ± 0.26 |
| 1.49 ± 0.34 | 0.062 ± 0.014 | 54.2 ± 15.0 | 0.89 ± 0.21 | 0.037 ± 0.008 | 45.8 ± 15.0 | 24 | 2.38 ± 0.55 | |
| MSN-maleamic Cu | 0.85 ± 0.06 | 0.141 ± 0.010 | 75.8 ± 10.6 | 0.23 ± 0.12 | 0.012 ± 0.001 | 24.2 ± 10.6 | 6 | 1.08 ± 0.18 |
| 0.56 ± 0.14 | 0.023 ± 0.006 | 70.1 ± 10.9 | 0.31 ± 0.19 | 0.009 ± 0.005 | 29.9 ± 10.9 | 24 | 0.87 ± 0.33 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-García, D.; Ardiles, P.R.; Prashar, S.; Rodríguez-Diéguez, A.; Páez, P.L.; Gómez-Ruiz, S. Preparation and Study of the Antibacterial Applications and Oxidative Stress Induction of Copper Maleamate-Functionalized Mesoporous Silica Nanoparticles. Pharmaceutics 2019, 11, 30. https://doi.org/10.3390/pharmaceutics11010030
Díaz-García D, Ardiles PR, Prashar S, Rodríguez-Diéguez A, Páez PL, Gómez-Ruiz S. Preparation and Study of the Antibacterial Applications and Oxidative Stress Induction of Copper Maleamate-Functionalized Mesoporous Silica Nanoparticles. Pharmaceutics. 2019; 11(1):30. https://doi.org/10.3390/pharmaceutics11010030
Chicago/Turabian StyleDíaz-García, Diana, Perla R. Ardiles, Sanjiv Prashar, Antonio Rodríguez-Diéguez, Paulina L. Páez, and Santiago Gómez-Ruiz. 2019. "Preparation and Study of the Antibacterial Applications and Oxidative Stress Induction of Copper Maleamate-Functionalized Mesoporous Silica Nanoparticles" Pharmaceutics 11, no. 1: 30. https://doi.org/10.3390/pharmaceutics11010030