Process Optimization and Upscaling of Spray-Dried Drug-Amino acid Co-Amorphous Formulations
Abstract
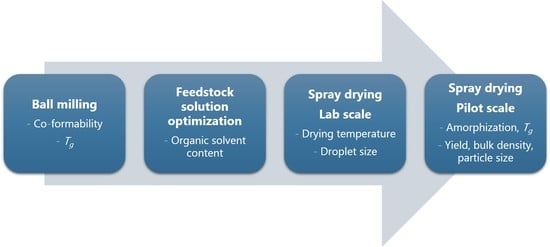
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Co-Amorphous Formulations at Laboratory Scale SD
2.2.1.1. Feedstock Solution Preparation
2.2.1.2. Spray Drying at Laboratory Scale
2.2.1.3. Experimental Design
2.2.2. Analytical Techniques
X-ray Powder Diffraction (XRPD)
Differential Scanning Calorimetry (DSC)
Bulk Density
Particle Size Distribution
2.2.3. Upscaling from Laboratory Scale to Pilot Scale SD
3. Results
3.1. Characterization of Formulations Produced in a Laboratory Scale Spray Dryer
3.2. Model Statistics
3.3. Overall Model
3.4. Upscaling of Selected Formulations to Pilot Scale Spray Dryer
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Babu, N.J.; Nangia, A. Solubility Advantage of Amorphous Drugs and Pharmaceutical Cocrystals. Crystal Growth Des. 2011, 11, 2662–2679. [Google Scholar] [CrossRef]
- Amidon, G.L.; Lennernäs, H.; Shah, V.P.; Crison, J.R. A Theoretical Basis for a Biopharmaceutic Drug Classification: The Correlation of in Vitro Drug Product Dissolution and in Vivo Bioavailability. Pharm. Res. 1995, 12, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug solubility: Importance and enhancement techniques. ISRN Pharm. 2012, 2012, 195727. [Google Scholar] [CrossRef] [PubMed]
- Hancock, B.C.; Zografi, G. Characteristics and significance of the amorphous state in pharmaceutical systems. J. Pharm. Sci. 1997, 86, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bikiaris, D.N. Solid dispersions, part I: Recent evolutions and future opportunities in manufacturing methods for dissolution rate enhancement of poorly water-soluble drugs. Expert Opin. Drug Deliv. 2011, 8, 1501–1519. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, Y.; Wada, K.; Nakatani, M.; Yamada, S.; Onoue, S. Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: Basic approaches and practical applications. Int. J. Pharm. 2011, 420, 1–10. [Google Scholar] [CrossRef]
- Alleso, M.; Chieng, N.; Rehder, S.; Rantanen, J.; Rades, T.; Aaltonen, J. Enhanced dissolution rate and synchronized release of drugs in binary systems through formulation: Amorphous naproxen-cimetidine mixtures prepared by mechanical activation. J. Control Release 2009, 136, 45–53. [Google Scholar] [CrossRef]
- Chieng, N.; Aaltonen, J.; Saville, D.; Rades, T. Physical characterization and stability of amorphous indomethacin and ranitidine hydrochloride binary systems prepared by mechanical activation. Eur. J. Pharm. Biopharm. 2009, 71, 47–54. [Google Scholar] [CrossRef]
- Dengale, S.J.; Ranjan, O.P.; Hussen, S.S.; Krishna, B.S.; Musmade, P.B.; Gautham Shenoy, G.; Bhat, K. Preparation and characterization of co-amorphous Ritonavir-Indomethacin systems by solvent evaporation technique: Improved dissolution behavior and physical stability without evidence of intermolecular interactions. Eur. J. Pharm. Sci. 2014, 62, 57–64. [Google Scholar] [CrossRef]
- Lobmann, K.; Grohganz, H.; Laitinen, R.; Strachan, C.; Rades, T. Amino acids as co-amorphous stabilizers for poorly water soluble drugs—Part 1: Preparation, stability and dissolution enhancement. Eur. J. Pharm. Biopharm. 2013, 85, 873–881. [Google Scholar] [CrossRef]
- Dengale, S.J.; Grohganz, H.; Rades, T.; Lobmann, K. Recent advances in co-amorphous drug formulations. Adv. Drug Deliv. Rev. 2016, 100, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Wickstrom, H.; Palo, M.; Rijckaert, K.; Kolakovic, R.; Nyman, J.O.; Maattanen, A.; Ihalainen, P.; Peltonen, J.; Genina, N.; de Beer, T.; et al. Improvement of dissolution rate of indomethacin by inkjet printing. Eur. J. Pharm. Sci. 2015, 75, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Löbmann, K.; Schnitzkewitz, J.; Knuhtsen, A.; Pedersen, D.S.; Rades, T.; Grohganz, H. Dipeptides as co-formers in co-amorphous systems. Eur. J. Pharm. Biopharm. 2019, 134, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Descamps, M.; Willart, J.F.; Dudognon, E.; Caron, V. Transformation of pharmaceutical compounds upon milling and comilling: The role of T(g). J. Pharm. Sci. 2007, 96, 1398–1407. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, R.; Lobmann, K.; Grohganz, H.; Strachan, C.; Rades, T. Amino acids as co-amorphous excipients for simvastatin and glibenclamide: Physical properties and stability. Mol. Pharm. 2014, 11, 2381–2389. [Google Scholar] [CrossRef]
- Lenz, E.; Jensen, K.T.; Blaabjerg, L.I.; Knop, K.; Grohganz, H.; Lobmann, K.; Rades, T.; Kleinebudde, P. Solid-state properties and dissolution behaviour of tablets containing co-amorphous indomethacin-arginine. Eur. J. Pharm. Biopharm. 2015, 96, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.T.; Lobmann, K.; Rades, T.; Grohganz, H. Improving co-amorphous drug formulations by the addition of the highly water soluble amino Acid, proline. Pharmaceutics 2014, 6, 416–435. [Google Scholar] [CrossRef]
- Karagianni, A.; Kachrimanis, K.; Nikolakakis, I. Co-Amorphous Solid Dispersions for Solubility and Absorption Improvement of Drugs: Composition, Preparation, Characterization and Formulations for Oral Delivery. Pharmaceutics 2018, 10, 98. [Google Scholar] [CrossRef]
- Lenz, E.; Lobmann, K.; Rades, T.; Knop, K.; Kleinebudde, P. Hot Melt Extrusion and Spray Drying of Co-amorphous Indomethacin-Arginine With Polymers. J. Pharm. Sci. 2017, 106, 302–312. [Google Scholar] [CrossRef]
- Sosnik, A.; Seremeta, K.P. Advantages and challenges of the spray-drying technology for the production of pure drug particles and drug-loaded polymeric carriers. Adv. Colloid Interface Sci. 2015, 223, 40–54. [Google Scholar] [CrossRef]
- Kasten, G.; Nouri, K.; Grohganz, H.; Rades, T.; Lobmann, K. Performance comparison between crystalline and co-amorphous salts of indomethacin-lysine. Int. J. Pharm. 2017, 533, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Van den Mooter, G. Spray drying formulation of amorphous solid dispersions. Adv. Drug Deliv. Rev. 2016, 100, 27–50. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.T.; Blaabjerg, L.I.; Lenz, E.; Bohr, A.; Grohganz, H.; Kleinebudde, P.; Rades, T.; Lobmann, K. Preparation and characterization of spray-dried co-amorphous drug-amino acid salts. J. Pharm. Pharmacol. 2016, 68, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Mishra, J.; Lobmann, K.; Grohganz, H.; Rades, T. Influence of preparation technique on co-amorphization of carvedilol with acidic amino acids. Int. J. Pharm. 2018, 552, 407–413. [Google Scholar] [CrossRef]
- Chiou, W.L. Pharmaceutical applications of solid dispersion systems: X-ray diffraction and aqueous solubility studies on griseofulvin-polyethylene glycol 6000 systems. J. Pharm. Sci. 1977, 66, 989–991. [Google Scholar] [CrossRef]
- United States Pharmacopeial Convention. USP 30; United States Pharmacopeial Convention: Rockville, MD, USA, 2006. [Google Scholar]
- Gaspar, F.; Vicente, J.; Neves, F.; Authelin, J. Spray drying: Scale-up and Manufacturing. In Amorphous Solid Dispersions: Theory Practice; Shah, N., Sandhu, H., Choi, D.S., Chokshi, H., Malick, A.W., Eds.; Springer: New York, NY, USA, 2014. [Google Scholar]
- Yousefi, S.; Emam-Djomeh, Z.; Mousavi, S.M. Effect of carrier type and spray drying on the physicochemical properties of powdered and reconstituted pomegranate juice (Punica Granatum L.). J. Food Sci. Technol. 2011, 48, 677–684. [Google Scholar] [CrossRef]
- Politis, S.N.; Colombo, P.; Colombo, G.; Rekkas, D.M. Design of experiments (DoE) in pharmaceutical development. Drug Dev. Ind. Pharm. 2017, 43, 889–901. [Google Scholar] [CrossRef]
- Ingvarsson, P.T.; Yang, M.; Mulvad, H.; Nielsen, H.M.; Rantanen, J.; Foged, C. Engineering of an inhalable DDA/TDB liposomal adjuvant: A quality-by-design approach towards optimization of the spray drying process. Pharm. Res. 2013, 30, 2772–2784. [Google Scholar] [CrossRef]
- Baldinger, A.; Clerdent, L.; Rantanen, J.; Yang, M.; Grohganz, H. Quality by design approach in the optimization of the spray-drying process. Pharm. Dev. Technol. 2012, 17, 389–397. [Google Scholar] [CrossRef]
- Rumondor, A.C.; Taylor, L.S. Application of partial least-squares (PLS) modeling in quantifying drug crystallinity in amorphous solid dispersions. Int. J. Pharm. 2010, 398, 155–160. [Google Scholar] [CrossRef]
- Hancock, B.C.; Zografi, G. The Relationship Between the Glass Transition Temperature and the Water Content of Amorphous Pharmaceutical Solids. Pharm. Res. 1994, 11, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Anandharamakrishnan, C.; Padma Ishwarya, S. Spray Drying Techniques for Food Ingredient Encapsulation; John Wiley & Sons: Chicago, IL, USA, 2015. [Google Scholar]
- Walton, D. The morphology of spray-dried particles a qualitative view. Dry. Technol. 2000, 18, 1943–1986. [Google Scholar] [CrossRef]
- Paudel, A.; Worku, Z.A.; Meeus, J.; Guns, S.; Van den Mooter, G. Manufacturing of solid dispersions of poorly water soluble drugs by spray drying: Formulation and process considerations. Int. J. Pharm. 2013, 453, 253–284. [Google Scholar] [CrossRef] [PubMed]




| Formulation | Levels (Fatom, Tout) | |
|---|---|---|
| LS#1 | (+, −) | 1.4 kg/h, 55 °C |
| LS#2 | (−, −) | 0.5 kg/h, 55 °C |
| LS#3 | (0, 0) | 0.9 kg/h, 65 °C |
| LS#4 | (+, +) | 1.4 kg/h, 75 °C |
| LS#5 | (−, +) | 0.5 kg/h, 75 °C |
| Formulation | Tg (°C) (±SD) | Yield (%) | Bulk Density (g/cm3) (±SD) | Particle Size Distribution | |
|---|---|---|---|---|---|
| d50 (µm) (±SD) | Span (±SD) | ||||
| LS#1 | 96.16 (±0.18) | 52.7 | 0.37 (±0.01) | 2.88 (±0.69) | 6.81 (±0.18) |
| LS#2 | 100.34 (±3.20) | 50.6 | 0.31 (±0.01) | 2.12 (±0.01) | 4.47 (±0.22) |
| LS#3 | 99.04 (±0.67) | 65.0 | 0.29 (±0.01) | 4.23 (±0.36) | 3.71 (±0.22) |
| LS#4 | 99.35 (±1.25) | 64.4 | 0.28 (±0.01) | 2.71 (±0.03) | 6.03 (±0.10) |
| LS#5 | 102.72 (±4.44) | 96.8 | 0.25 (±0.01) | 3.19 (±0.12) | 2.33 (±0.02) |
| Formulation | Average Tg (°C) (±SD) | Yield (%) | Bulk Density (g/cm3) (±SD) | Particle Size Distribution | |
|---|---|---|---|---|---|
| d50 (µm) (±SD) | Span (±SD) | ||||
| PS#1 | 103.16 (±4.99) | 83.35 | 0.24 (±0.01) | 2.56 (±0.07) | 3.01 (±0.21) |
| PS#2 | 99.66 (±0.14) | 73.15 | 0.25 (±0.01) | 1.97 (±0.02) | 2.38 (±0.35) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasten, G.; Duarte, Í.; Paisana, M.; Löbmann, K.; Rades, T.; Grohganz, H. Process Optimization and Upscaling of Spray-Dried Drug-Amino acid Co-Amorphous Formulations. Pharmaceutics 2019, 11, 24. https://doi.org/10.3390/pharmaceutics11010024
Kasten G, Duarte Í, Paisana M, Löbmann K, Rades T, Grohganz H. Process Optimization and Upscaling of Spray-Dried Drug-Amino acid Co-Amorphous Formulations. Pharmaceutics. 2019; 11(1):24. https://doi.org/10.3390/pharmaceutics11010024
Chicago/Turabian StyleKasten, Georgia, Íris Duarte, Maria Paisana, Korbinian Löbmann, Thomas Rades, and Holger Grohganz. 2019. "Process Optimization and Upscaling of Spray-Dried Drug-Amino acid Co-Amorphous Formulations" Pharmaceutics 11, no. 1: 24. https://doi.org/10.3390/pharmaceutics11010024