Xylan-Based Hydrogels as a Potential Carrier for Drug Delivery: Effect of Pore-Forming Agents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagent and Apparatus
2.2. Synthesis of Carboxymethyl Xylan (CMX)
2.3. Peparation of Hydrogels
2.4. Characterization of Hydrogels
2.5. Swelling Behavior Studies of Hydrogels
2.6. Drug Release
2.6.1. Standard Curve of 5-Fu
2.6.2. Preparation of 5-Fu-Loaded Hydrogels
2.6.3. Drug Release of Hydrogels
3. Results and Discussion
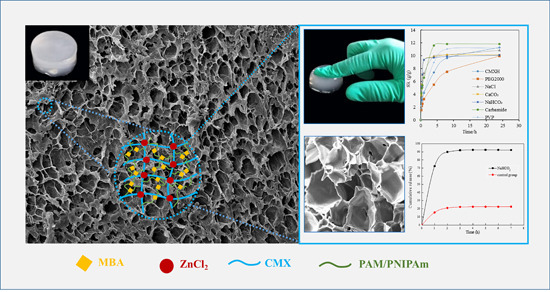
3.1. Pore-Making Mechanism for Hydrogels
3.2. FTIR Analysis of Hydrogels
3.3. Mechanical Properties Analysis of Hydrogels
3.4. TGA Analysis of Hydrogels
3.5. SEM Analysis of Hydrogels
3.6. Zn2+ Analysis of Hydrogels
3.7. Swelling Behavior Studies of Hydrogels
3.8. Drug Release of the Hydrogel Made with NaHCO3 Pore-Forming Agent
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rang, K.A.; Lee, S.L.; Park, S.N. Properties and in vitro drug release of pH- and temperature-sensitive double cross-linked interpenetrating polymer network hydrogels based on hyaluronic acid/poly(N-isopropylacrylamide) for transdermal delivery of luteolin. Int. J. Biol. Macromol. 2018, 118, 731–740. [Google Scholar] [CrossRef]
- Benavidez, T.E.; Baruzzi, A.M. Comparative behavior of glucose oxidase and oxalate oxidase immobilized in mucin/chitosan hydrogels for biosensors applications. Polymer 2012, 53, 438–444. [Google Scholar] [CrossRef]
- Seliktar, D. Designing cell-compatible hydrogels for biomedical applications. Science 2012, 336, 1124–1128. [Google Scholar] [CrossRef] [PubMed]
- Iamsaard, S.; Aßhoff, S.J.; Matt, B.; Kudernac, T.; Cornelissen, J.J.L.M.; Fletcher, S.P. Conversion of light into macroscopic helical motion. Nat. Chem. 2014, 6, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Kurashina, K.; Bruijn, J.D.D. A preliminary study on osteoinduction of two kinds of calcium phosphate ceramics. Biomaterials 1999, 20, 1799–1806. [Google Scholar] [CrossRef]
- Dey, A.; Bera, B.; Bera, R. Influence of diethylene glycol as a porogen in a glyoxal crosslinked polyvinyl alcohol hydrogel. RSC Adv. 2014, 4, 42260–42270. [Google Scholar] [CrossRef]
- Badiger, M.V.; Mcneill, M.E.; Graham, N.B. Porogens in the preparation of microporous hydrogels based on poly(ethylene oxides). Biomaterials 1993, 14, 1059–1063. [Google Scholar] [CrossRef]
- Lee, A.G.; Arena, C.P.; Beebe, D.J. Development of macroporous poly(ethylene glycol) hydrogel arrays within microfluidic channels. Biomacromolecules 2010, 11, 3316–3324. [Google Scholar] [CrossRef]
- Sergeeva, A.; Feoktistova, N.; Prokopovic, V. Design of porous alginate hydrogels by sacrificial CaCO3 templates: Pore formation mechanism. Adv. Mater. Interfaces 2016, 2, 1500386–1500395. [Google Scholar] [CrossRef]
- Gao, M.; Gawel, K.; Stokke, B. Swelling dynamics of a DNA-polymer hybrid hydrogel prepared using polyethylene glycol as a porogen. Gels 2015, 1, 219–234. [Google Scholar] [CrossRef]
- Kabiri, K.; Omidian, H.; Hashemi, S.A. Concise synthesis of fast-swelling superabsorbent hydrogels: Effect of initiator concentration on porosity and absorption rate. Eur. Polym. J. 2003, 39, 1341–1348. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Fahmy, A.; Taha, T.H. Thermo-and pH-sensitive hydrogel membranes composed of poly(N-isopropylacrylamide)-hyaluronan for biomedical applications: Influence of hyaluronan incorporation on the membrane properties. Int. J. Biol. Macromol. 2018, 106, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Sole, I.; Vilchez, S.; Miras, J.; Montanya, N. DHA and L-carnitine loaded chitosan hydrogels as delivery systems for topical applications. Colloids Surf. A Physicochem. Eng. Asp. 2017, 525, 85–92. [Google Scholar] [CrossRef]
- Deng, C.; Li, F.; Hackett, J.M.; Chaudhry, S.H.; Toll, F.N. Collagen and glycopolymer based hydrogel for potential corneal application. Acta Biomater. 2010, 6, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Takei, Y.G.; Aoki, T.; Sanui, K.; Ogata, N.; Okano, T.; Sakurai, Y. Temperature responsive bioconjugates. 2. Molecular design for temperature-modulated bioseparations. Bioconjug. Chem. 1993, 4, 341–346. [Google Scholar] [CrossRef]
- Wilkie, K.C.B. The hemicelluloses of grasses and cereals gramineae. Adv. Carbohydr. Chem. Biochem. 1979, 36, 215–264. [Google Scholar] [CrossRef]
- Oliveira, E.E.; Silva, A.E.; Junior, T.N.; Gomes, M.C.S.; Aguiar, L.M. Xylan from corn cobs, a promising polymer for drug delivery: Production and characterization. Bioresour. Technol. 2010, 101, 5402–5406. [Google Scholar] [CrossRef]
- Gao, C.; Ren, J.; Zhao, C. Xylan-based temperature/pH sensitive hydrogels for drug controlled release. Carbohydr. Polym. 2016, 151, 189–197. [Google Scholar] [CrossRef]
- Kong, W.Q.; Gao, C.D.; Hu, S.F. Xylan-modified-based hydrogels with temperature/pH dual sensitivity and controllable drug delivery behavior. Materials 2017, 10, 304. [Google Scholar] [CrossRef]
- Cao, X.; Peng, X.; Zhong, L. Multiresponsive hydrogels based on xylan-type hemicelluloses and photoisomerized azobenzene copolymer as drug delivery carrier. J. Agric. Food Chem. 2014, 62, 10000–10007. [Google Scholar] [CrossRef]
- Chimphango, A.F.A.; Zyl, W.H.V.; Görgens, J.F. In situ enzymatic aided formation of xylan hydrogels and encapsulation of horse radish peroxidase for slow release. Carbohydr. Polym. 2012, 88, 1109–1117. [Google Scholar] [CrossRef]
- Sun, X.F.; Wang, H.H.; Jing, Z.X.; Mohanathas, R. Hemicellulose-based pH-sensitive and biodegradable hydrogel for controlled drug delivery. Carbohydr. Polym. 2013, 92, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Kouvaraki, M.A.; Ajani, J.P.; Wolff, R. Fluorouracil, doxorubicin, and streptozocin in the treatment of patients with locally advanced and metastatic pancreatic endocrine carcinomas. J. Clin. Oncol. 2004, 22, 4762–4771. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, S.J.; Begg, E.J.; Robinson, B.A. The effect of dihydropyrimidine dehydrogenase deficiency on outcomes with fluorouracil. Advers. Drug React. Toxicol. Rev. 2002, 21, 1–16. [Google Scholar] [CrossRef]
- Singh, B.; Chauhan, N. Preliminary evaluation of molecular imprinting of 5-fluorouracil within hydrogels for use as drug delivery systems. Acta Biomater. 2008, 4, 1244–1254. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.W.; Ren, J.L.; Zhong, L.X. Microwave-induced synthesis of carboxymethyl hemicelluloses and their rheological properties. J. Agric. Food. Chem. 2011, 59, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Kwon, K.; Auh, J.H.; Kim, J.W. Physicochemical properties and functionality of highly carboxymethylated starch. Starch Starke 2010, 49, 499–505. [Google Scholar] [CrossRef]
- Deng, C.; Han, X.; Li, R.; Zhang, Q. Preparation and in vitro drug release of 5-Fu in Poly(N-isopropylacrylamide-co-acrylate acid) hydrogel. Acta Acad. Med. CPAF 2010, 19, 695–697. [Google Scholar]
- Kong, W.; Huang, D.; Xu, G. A new design strategy for graphene oxide/polyacrylamide/aluminium ion-crosslinked carboxymethyl hemicelluloses nanocomposite hydrogels with highly tough and elastic properties. Chem. Asian J. 2016, 11, 1697–1704. [Google Scholar] [CrossRef]
- Peng, X.W.; Ren, J.L.; Zhong, L.X.; Peng, F.; Sun, R.C. Xylan-rich hemicelluloses-graft-acrylic acid ionic hydrogels with rapid responses to pH, salt, and organic solvents. J. Agric. Food. Chem. 2011, 59, 8208–8215. [Google Scholar] [CrossRef]
- Gao, C.D.; Ren, J.L.; Kong, W.Q.; Sun, R.C.; Chen, Q.F. Comparative study on temperature/pH sensitive xylan-based hydrogels: Their properties and drug controlled release. RSC Adv. 2015, 5, 90671–90681. [Google Scholar] [CrossRef]
- Wei, Q.B.; Fu, F.; Zhang, Y.Q. pH-responsive CMC/PAM/PVP semi-IPN hydrogels for theophylline drug release. J. Polym. Res. 2014, 21, 453–462. [Google Scholar] [CrossRef]
- Patra, S.K.; Swain, S.K. Swelling study of superabsorbent PAA-co-PAM/clay nanohydrogel. J. Appl. Polym. Sci. 2011, 120, 533–1538. [Google Scholar] [CrossRef]
- El-Mohdy, H.L.A. Water sorption behavior of CMC/PAM hydrogels prepared by γ-irradiation and release of potassium nitrate as agrochemical. React. Funct. Polym. 2007, 67, 1094–1102. [Google Scholar] [CrossRef]
- Okay, O. Macroporous copolymer networks. Prog. Polym. Sci. 2000, 25, 711–779. [Google Scholar] [CrossRef]
- Tang, Q.; Sun, X.; Li, Q. Preparation of porous polyacrylate/poly(ethylene glycol) interpenetrating network hydrogel and simplification of Flory theory. J. Mater. Sci. 2009, 44, 3712–3718. [Google Scholar] [CrossRef]
- Ma, J.; Li, Z.; Zhen, L. Preparation and characterization of porous poly(N-isopropylacrylamide)/clay nanocomposite hydrogels. Polym. Bull. 2008, 61, 593–602. [Google Scholar] [CrossRef]
- Zhang, E.; Yin, D.; Xu, L. Microstructure, mechanical and corrosion properties and biocompatibility of Mg-Zn-Mn alloys for biomedical application. Mater. Sci. Eng. C 2009, 29, 987–993. [Google Scholar] [CrossRef]
- El-Din, H.M.N.; Alla, S.G.A.; El-Naggar, A.W.M. Swelling and drug release properties of acrylamide/carboxymethyl cellulose networks formed by gamma irradiation. Radiat. Phys. Chem. 2010, 79, 725–730. [Google Scholar] [CrossRef]
- Sun, X.F.; Jing, Z.; Wang, G. Preparation and swelling behaviors of porous hemicellulose-g-polyacrylamide hydrogels. J. Appl. Polym. Sci. 2013, 128, 1861–1870. [Google Scholar] [CrossRef]








| Sample | Pore-Forming Agent | Washing Method |
|---|---|---|
| gel-1 | — | Soaked with ultra-pure water for one week |
| gel-2 | PEG2000 | Soaked with ethanol for three days then ultra-pure water for four days |
| gel-3 | NaCl | Soaked with ultra-pure water for one week |
| gel-4 | CaCO3 | Soaked with HCl solution for three days then ultra-pure water for four days |
| gel-5 | NaHCO3 | Soaked with HCl solution for three days then ultra-pure water for four days |
| gel-6 | carbamide | Soaked with ultra-pure water for one week |
| gel-7 | PVP | Soaked with ultra-pure water for one week |
| Sample | gel-1 | gel-2 | gel-3 | gel-4 | gel-5 | gel-6 | gel-7 |
|---|---|---|---|---|---|---|---|
| Hydrogel Weight (mg) | 33.80 | 50.80 | 71.50 | 43.40 | 63.90 | 43.60 | 51.50 |
| Zinc Concentration (mg/L) | 7.18 | 15.02 | 14.44 | 4.17 | 11.32 | 5.78 | 14.28 |
| Weight Percent of Zinc (%) | 0.21 | 0.29 | 0.20 | 0.09 | 0.18 | 0.13 | 0.27 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, M.; Liu, X.; Meng, L.; Wang, X.; Ren, J. Xylan-Based Hydrogels as a Potential Carrier for Drug Delivery: Effect of Pore-Forming Agents. Pharmaceutics 2018, 10, 261. https://doi.org/10.3390/pharmaceutics10040261
Chang M, Liu X, Meng L, Wang X, Ren J. Xylan-Based Hydrogels as a Potential Carrier for Drug Delivery: Effect of Pore-Forming Agents. Pharmaceutics. 2018; 10(4):261. https://doi.org/10.3390/pharmaceutics10040261
Chicago/Turabian StyleChang, Minmin, Xinxin Liu, Ling Meng, Xiaohui Wang, and Junli Ren. 2018. "Xylan-Based Hydrogels as a Potential Carrier for Drug Delivery: Effect of Pore-Forming Agents" Pharmaceutics 10, no. 4: 261. https://doi.org/10.3390/pharmaceutics10040261