Complex Polymeric Architectures Self-Assembling in Unimolecular Micelles: Preparation, Characterization and Drug Nanoencapsulation
Abstract
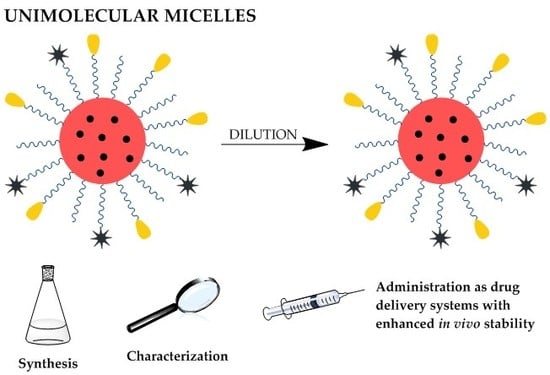
:1. Introduction
2. Preparation of Unimolecular Micelles
2.1. Multi-Arm Polymers
2.2. Hyperbranched Polymers
3. Characterization Methods
3.1. Characterization of Unimolecular Micelles
3.2. Characterization of Multi-Micelle Aggregates
4. Unimolecular Micelles as Drug Delivery Systems
4.1. Drug Loading and Release
4.2. Biocompatibility
4.3. Multi-Arm Copolymers for Drug Delivery
4.4. Other Hyperbranched Copolymers
5. Conclusions
Funding
Conflicts of Interest
References
- Fan, X.; Li, Z.; Loh, X.J. Recent development of unimolecular micelles as functional materials and applications. Polym. Chem. 2016, 7, 5898–5919. [Google Scholar] [CrossRef]
- Thota, B.N.S.; Urner, L.H.; Haag, R. Supramolecular Architectures of Dendritic Amphiphiles in Water. Chem. Rev. 2016, 116, 2079–2102. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhao, T.; Zhu, X.; Yan, D.; Wang, W. Bioapplications of hyperbranched polymers. Chem. Soc. Rev. 2015, 44, 4023–4071. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Yu, H.; Xinyuan, Z. Fluorescent Unimolecular Conjugated Polymeric Micelles for Biological Applications. Macromol. Chem. Phys. 2016, 217, 266–283. [Google Scholar]
- Zhou, Y.; Huang, W.; Liu, J.; Zhu, X.; Yan, D. Self-Assembly of Hyperbranched Polymers and Its Biomedical Applications. Adv. Mater. 2010, 22, 4567–4590. [Google Scholar] [CrossRef] [PubMed]
- Attwood, D.; Booth, C.; Yeates, S.G.; Chaibundit, C.; Ricardo, N.M.P.S. Block copolymers for drug solubilisation: Relative hydrophobicities of polyether and polyester micelle-core-forming blocks. Int. J. Pharm. 2007, 345, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lin, W.; Wen, L.; Yao, N.; Nie, S.; Zhang, L. Systematic design and application of unimolecular star-like block copolymer micelles: A coarse-grained simulation study. Phys. Chem. Chem. Phys. 2016, 18, 26519–26529. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K. Functional dendrimers, hyperbranched and star polymers. Prog. Polym. Sci. 2000, 25, 453–571. [Google Scholar] [CrossRef]
- Zamurovic, M.; Christodoulou, S.; Vazaios, A.; Iatrou, E.; Pitsikalis, M.; Hadjichristidis, N. Micellization Behavior of Complex Comblike Block Copolymer Architectures. Macromolecules 2007, 40, 5835–5849. [Google Scholar] [CrossRef]
- Knop, K.; Pavlov, G.M.; Rudolph, T.; Martin, K.; Pretzel, D.; Jahn, B.O.; Scharf, D.H.; Brakhage, A.A.; Makarov, V.; Mollmann, U.; et al. Amphiphilic star-shaped block copolymers as unimolecular drug delivery systems: Investigations using a novel fungicide. Soft Matter 2013, 9, 715–726. [Google Scholar] [CrossRef]
- Haag, R. Supramolecular Drug-Delivery Systems Based on Polymeric Core–Shell Architectures. Angew. Chem. Int. Ed. 2003, 43, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Patri, A.K.; Majoros, I.J.; Baker, J.R. Dendritic polymer macromolecular carriers for drug delivery. Curr. Opin. Chem. Boil. 2002, 6, 466–471. [Google Scholar] [CrossRef]
- Gao, H. Development of Star Polymers as Unimolecular Containers for Nanomaterials. Macromol. Rapid Commun. 2012, 33, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Shi, Y.; Kim, J.Y.; Park, K.; Cheng, J.-X. Overcoming the barriers in micellar drug delivery: Loading efficiency, in vivo stability, and micelle–cell interaction. Expert Opin. Drug Deliv. 2010, 7, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Farrell, S.; Uhrich, K. Drug release characteristics of unimolecular polymeric micelles. J. Control. Release 2000, 68, 167–174. [Google Scholar] [CrossRef]
- Liu, X.; Fan, X.; Jiang, L.; Loh, X.J.; Wu, Y.-L.; Li, Z. Biodegradable polyester unimolecular systems as emerging materials for therapeutic applications. J. Mater. Chem. B 2018, 6, 5488–5498. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, H.B.; Sun, Z.Y.; Li, H.A.; Huang, H.H.; Liu, L.X.; Chen, Y.M. Shell of amphiphilic molecular bottlebrush matters as unimolecular micelle. Polymer 2018, 149, 316–324. [Google Scholar] [CrossRef]
- Li, H.A.; Liu, H.; Nie, T.Q.; Chen, Y.; Wang, Z.Y.; Huang, H.H.; Liu, L.X.; Chen, Y.M. Molecular bottlebrush as a unimolecular vehicle with tunable shape for photothermal cancer therapy. Biomaterials 2018, 178, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Polovnikov, K.E.; Potemkin, I.I. Effect of Architecture on Micelle Formation and Liquid-Crystalline Ordering in Solutions of Block Copolymers Comprising Flexible and Rigid Blocks: Rod-Coil vs. Y-Shaped vs. Comblike Copolymers. J. Phys. Chem. B 2017, 121, 10180–10189. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.S.; Wang, X.Y.; Cao, M.Y.; Wang, C.G.; Hu, Z.G.; Wu, Y.L.; Li, Z.B.; Loh, X.J. “Y”-shape armed amphiphilic star-like copolymers: Design, synthesis and dual-responsive unimolecular micelle formation for controlled drug delivery. Polym. Chem. 2017, 8, 5611–5620. [Google Scholar] [CrossRef]
- Tu, X.Y.; Meng, C.; Wang, Y.F.; Ma, L.W.; Wang, B.Y.; He, J.L.; Ni, P.H.; Ji, X.L.; Liu, M.Z.; Wei, H. Fabrication of Thermosensitive Cyclic Brush Copolymer with Enhanced Therapeutic Efficacy for Anticancer Drug Delivery. Macromol. Rapid Commun. 2018, 39. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J.; Pitto-Barry, A.; Kirby, N.; Dove, A.P.; O’Reilly, R.K. Cyclic Graft Copolymer Unimolecular Micelles: Effects of Cyclization on Particle Morphology and Thermoresponsive Behavior. Macromolecules 2016, 49, 2802–2813. [Google Scholar] [CrossRef] [PubMed]
- Hadjichristidis, N.; Iatrou, H.; Pitsikalis, M.; Mays, J. Macromolecular architectures by living and controlled/living polymerizations. Prog. Polym. Sci. 2006, 31, 1068–1132. [Google Scholar] [CrossRef]
- Yang, X.Q.; Grailer, J.J.; Pilla, S.; Steeber, D.A.; Gong, S.Q. Tumor-Targeting, pH-Responsive, and Stable Unimolecular Micelles as Drug Nanocarriers for Targeted Cancer Therapy. Bioconjug. Chem. 2010, 21, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wang, L.; Cordie, T.; Vokoun, C.; Eliceiri, K.W.; Gong, S. Multi-functional self-fluorescent unimolecular micelles for tumor-targeted drug delivery and bioimaging. Biomaterials 2015, 47, 41–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.R.; Zhao, X.Z.; Zhang, X.L.; Ma, L.W.; Wang, B.Y.; Wei, H. Optimization of bioreducible micelles self-assembled from amphiphilic hyperbranched block copolymers for drug delivery. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 1383–1394. [Google Scholar] [CrossRef]
- Yin, M.; Ding, K.; Gropeanu, R.A.; Shen, J.; Berger, R.; Weil, T.; Müllen, K. Dendritic Star Polymers for Efficient DNA Binding and Stimulus-Dependent DNA Release. Biomacromolecules 2008, 9, 3231–3238. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Jaskula–Sztul, R.; Harrison, A.; Dammalapati, A.; Xu, W.; Cheng, Y.; Chen, H.; Gong, S. KE108-conjugated unimolecular micelles loaded with a novel HDAC inhibitor thailandepsin-A for targeted neuroendocrine cancer therapy. Biomaterials 2016, 97, 22–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Jiang, A.; Guo, J.; Uhrich Kathryn, E. Unimolecular micelles: Synthesis and characterization of amphiphilic polymer systems. J. Polym. Sci. Part A Polym. Chem. 2000, 37, 703–711. [Google Scholar] [CrossRef]
- Schramm, O.G.; Meier, M.A.R.; Hoogenboom, R.; van Erp, H.P.; Gohy, J.-F.; Schubert, U.S. Polymeric nanocontainers with high loading capacity of hydrophobic drugs. Soft Matter 2009, 5, 1662–1667. [Google Scholar] [CrossRef]
- Bruni, R.; Possenti, P.; Bordignon, C.; Li, M.; Ordanini, S.; Messa, P.; Rastaldi, M.P.; Cellesi, F. Ultrasmall polymeric nanocarriers for drug delivery to podocytes in kidney glomerulus. J. Control. Release 2017, 255, 94–107. [Google Scholar] [CrossRef] [PubMed]
- Krämer, M.; Stumbé, J.F.; Türk, H.; Krause, S.; Komp, A.; Delineau, L.; Prokhorova, S.; Kautz, H.; Haag, R. pH-Responsive Molecular Nanocarriers Based on Dendritic Core-Shell Architectures. Angew. Chem. Int. Ed. 2002, 41, 4252–4256. [Google Scholar] [CrossRef]
- Kumar, K.R.; Brooks, D.E. Comparison of Hyperbranched and Linear Polyglycidol Unimolecular Reverse Micelles as Nanoreactors and Nanocapsules. Macromol. Rapid Commun. 2005, 26, 155–159. [Google Scholar] [CrossRef]
- Qiu, L.; Liu, Q.; Hong, C.-Y.; Pan, C.-Y. Unimolecular micelles of camptothecin-bonded hyperbranched star copolymers via [small beta]-thiopropionate linkage: Synthesis and drug delivery. J. Mater. Chem. B 2016, 4, 141–151. [Google Scholar] [CrossRef]
- Du, W.; Nyström, A.M.; Zhang, L.; Powell, K.T.; Li, Y.; Cheng, C.; Wickline, S.A.; Wooley, K.L. Amphiphilic Hyperbranched Fluoropolymers as Nanoscopic 19F Magnetic Resonance Imaging Agent Assemblies. Biomacromolecules 2008, 9, 2826–2833. [Google Scholar] [CrossRef] [PubMed]
- Prabaharan, M.; Grailer, J.J.; Pilla, S.; Steeber, D.A.; Gong, S.Q. Folate-conjugated amphiphilic hyperbranched block copolymers based on Boltorn (R.) H40, poly(l-lactide) and poly(ethylene glycol) for tumor-targeted drug delivery. Biomaterials 2009, 30, 3009–3019. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, X.-Z.; Cheng, S.-X.; Zhuo, R.-X.; Gu, Z.-W. Functionalized Amphiphilic Hyperbranched Polymers for Targeted Drug Delivery. Biomacromolecules 2008, 9, 2578–2585. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Sun, P.; Tong, G.S.; Zhu, X.Y. Star polymer-based unimolecular micelles and their application in bio-imaging and diagnosis. Biomaterials 2018, 178, 738–750. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.Y.; Szymusiak, M.; Garcia, E.A.; Lock, L.L.; Cui, H.G.; Liu, Y.; Herrera-Alonso, M. Solute-Triggered Morphological Transitions of an Amphiphilic Heterografted Brush Copolymer as a Single-Molecule Drug Carrier. Macromolecules 2017, 50, 2201–2206. [Google Scholar] [CrossRef]
- Pesek, S.L.; Li, X.; Hammouda, B.; Hong, K.; Verduzco, R. Small-Angle Neutron Scattering Analysis of Bottlebrush Polymers Prepared via Grafting-Through Polymerization. Macromolecules 2013, 46, 6998–7005. [Google Scholar] [CrossRef]
- Bryant, G.; Thomas, J.C. Improved Particle Size Distribution Measurements Using Multiangle Dynamic Light Scattering. Langmuir 1995, 11, 2480–2485. [Google Scholar] [CrossRef]
- Yun, J.; Faust, R.; Szilágyi, L.S.; Kéki, S.; Zsuga, M. Effect of Architecture on the Micellar Properties of Amphiphilic Block Copolymers: Comparison of AB Linear Diblock, AAB, and A2B Heteroarm Star Block Copolymers. Macromolecules 2003, 36, 1717–1723. [Google Scholar] [CrossRef]
- Kwon, G.; Naito, M.; Yokoyama, M.; Okano, T.; Sakurai, Y.; Kataoka, K. Micelles based on AB block copolymers of poly(ethylene oxide) and poly(.beta.-benzyl L.-aspartate). Langmuir 1993, 9, 945–949. [Google Scholar] [CrossRef]
- Gao, X.; Wang, B.; Wei, X.; Rao, W.; Ai, F.; Zhao, F.; Men, K.; Yang, B.; Liu, X.; Huang, M.; et al. Preparation, characterization and application of star-shaped PCL/PEG micelles for the delivery of doxorubicin in the treatment of colon cancer. Int. J. Nanomed. 2013, 8, 971–982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Bronich, T.K.; Kabanov, A.V.; Rauh, R.D.; Roovers, J. Synthesis and Evaluation of a Star Amphiphilic Block Copolymer from Poly(ε-caprolactone) and Poly(ethylene glycol) as a Potential Drug Delivery Carrier. Bioconjug. Chem. 2005, 16, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Shibata, M.; Matsumoto, M.; Hirai, Y.; Takenaka, M.; Sawamoto, M.; Terashima, T. Intramolecular Folding or Intermolecular Self-Assembly of Amphiphilic Random Copolymers: On-Demand Control by Pendant Design. Macromolecules 2018, 51, 3738–3745. [Google Scholar] [CrossRef]
- Hong, H.; Mai, Y.; Zhou, Y.; Yan, D.; Cui, J. Self-Assembly of Large Multimolecular Micelles from Hyperbranched Star Copolymers. Macromol. Rapid Commun. 2007, 28, 591–596. [Google Scholar] [CrossRef]
- Radowski Michał, R.; Shukla, A.; von Berlepsch, H.; Böttcher, C.; Pickaert, G.; Rehage, H.; Haag, R. Supramolecular Aggregates of Dendritic Multishell Architectures as Universal Nanocarriers. Angew. Chem. Int. Ed. 2007, 46, 1265–1269. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Tu, C.; Chen, Y.; Shi, Y.; Song, L.; Wang, R.; Zhu, X.; Zhu, B.; Yan, D.; Han, T. Control of the Optical Properties of a Star Copolymer with a Hyperbranched Conjugated Polymer Core and Poly(ethylene glycol) Arms by Self-Assembly. Chem. A Eur. J. 2010, 16, 12710–12717. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Wang, D.; Zhu, Q.; Zhu, L.; Tong, G.; Lu, Y.; Yan, D.; Zhu, X. Real-Time Monitoring of Anticancer Drug Release with Highly Fluorescent Star-Conjugated Copolymer as a Drug Carrier. Biomacromolecules 2014, 15, 1355–1364. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Tu, C.; Wang, R.; Zhu, L.; Chen, Y.; Tong, G.; Zhu, B.; He, L.; Yan, D.; Zhu, X. Emission enhancement of conjugated polymers through self-assembly of unimolecular micelles to multi-micelle aggregates. Chem. Commun. 2011, 47, 9678–9680. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Ling, P.; Zhang, T. Polymeric Micelles, a Promising Drug Delivery System to Enhance Bioavailability of Poorly Water-Soluble Drugs. J. Drug Deliv. 2013, 2013, 340315. [Google Scholar] [CrossRef] [PubMed]
- Gullotti, E.; Yeo, Y. Extracellularly Activated Nanocarriers: A New Paradigm of Tumor Targeted Drug Delivery. Mol. Pharm. 2009, 6, 1041–1051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byrne, J.D.; Betancourt, T.; Brannon-Peppas, L. Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv. Drug Deliv. Rev. 2008, 60, 1615–1626. [Google Scholar] [CrossRef] [PubMed]
- Kowalczuk, A.; Trzcinska, R.; Trzebicka, B.; Müller, A.H.E.; Dworak, A.; Tsvetanov, C.B. Loading of polymer nanocarriers: Factors, mechanisms and applications. Prog. Polym. Sci. 2014, 39, 43–86. [Google Scholar] [CrossRef]
- Gaucher, G.; Dufresne, M.-H.; Sant, V.P.; Kang, N.; Maysinger, D.; Leroux, J.-C. Block copolymer micelles: Preparation, characterization and application in drug delivery. J. Control. Release 2005, 109, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.; van Colen, G.; Sander, B.; Golas, M.M.; Uezguen, S.; Weigandt, M.; Goepferich, A. Drug Loading of Polymeric Micelles. Pharm. Res. 2013, 30, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Mugabe, C.; Hadaschik Boris, A.; Kainthan Rajesh, K.; Brooks Donald, E.; So Alan, I.; Gleave Martin, E.; Burt Helen, M. Paclitaxel incorporated in hydrophobically derivatized hyperbranched polyglycerols for intravesical bladder cancer therapy. BJU Int. 2009, 103, 978–986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abouelmagd, S.A.; Sun, B.; Chang, A.C.; Ku, Y.J.; Yeo, Y. Release Kinetics Study of Poorly Water-Soluble Drugs from Nanoparticles: Are We Doing It Right? Mol. Pharm. 2015, 12, 997–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naahidi, S.; Jafari, M.; Edalat, F.; Raymond, K.; Khademhosseini, A.; Chen, P. Biocompatibility of engineered nanoparticles for drug delivery. J. Control. Release 2013, 166, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Ai, J.; Biazar, E.; Jafarpour, M.; Montazeri, M.; Majdi, A.; Aminifard, S.; Zafari, M.; Akbari, H.R.; Rad, H.G. Nanotoxicology and nanoparticle safety in biomedical designs. Int. J. Nanomed. 2011, 6, 1117–1127. [Google Scholar]
- Nachtergael, A.; Coulembier, O.; Dubois, P.; Helvenstein, M.; Duez, P.; Blankert, B.; Mespouille, L. Organocatalysis Paradigm Revisited: Are Metal-Free Catalysts Really Harmless? Biomacromolecules 2015, 16, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Tsarevsky, N.V.; Matyjaszewski, K. “Green” Atom Transfer Radical Polymerization: From Process Design to Preparation of Well-Defined Environmentally Friendly Polymeric Materials. Chem. Rev. 2007, 107, 2270–2299. [Google Scholar] [CrossRef] [PubMed]
- Proietti Silvestri, I.; Cellesi, F. AGET ATRP of Poly[poly(ethylene glycol) methyl ether methacrylate] Catalyzed by Hydrophobic Iron(III)–Porphyrins. Macromol. Chem. Phys. 2015, 216, 2032–2039. [Google Scholar] [CrossRef]
- Jiang, X.; Lok, M.C.; Hennink, W.E. Degradable-Brushed pHEMA–pDMAEMA Synthesized via ATRP and Click Chemistry for Gene Delivery. Bioconjug. Chem. 2007, 18, 2077–2084. [Google Scholar] [CrossRef] [PubMed]
- Atzet, S.; Curtin, S.; Trinh, P.; Bryant, S.; Ratner, B. Degradable poly(2-hydroxyethyl methacrylate)-co-polycaprolactone Hydrogels for Tissue Engineering Scaffolds. Biomacromolecules 2008, 9, 3370–3377. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.E.; Szoka, F.C.; Fréchet, J.M.J. Soluble Polymer Carriers for the Treatment of Cancer: The Importance of Molecular Architecture. Acc. Chem. Res. 2009, 42, 1141–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khanna, P.; Ong, C.; Bay, H.B.; Baeg, H.G. Nanotoxicity: An Interplay of Oxidative Stress, Inflammation and Cell Death. Nanomaterials 2015, 5, 1163–1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markovsky, E.; Baabur-Cohen, H.; Eldar-Boock, A.; Omer, L.; Tiram, G.; Ferber, S.; Ofek, P.; Polyak, D.; Scomparin, A.; Satchi-Fainaro, R. Administration, distribution, metabolism and elimination of polymer therapeutics. J. Control. Release 2012, 161, 446–460. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qian, Y.; Liu, T.; Hu, X.; Zhang, G.; You, Y.; Liu, S. Amphiphilic multiarm star block copolymer-based multifunctional unimolecular micelles for cancer targeted drug delivery and MR imaging. Biomaterials 2011, 32, 6595–6605. [Google Scholar] [CrossRef] [PubMed]
- Jaskula-Sztul, R.; Xu, W.; Chen, G.; Harrison, A.; Dammalapati, A.; Nair, R.; Cheng, Y.; Gong, S.; Chen, H. Thailandepsin A-loaded and octreotide-functionalized unimolecular micelles for targeted neuroendocrine cancer therapy. Biomaterials 2016, 91, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, X.; Tao, W.; Wang, Z.; Zhang, X.; Zhu, H.; Wu, Y.; Gao, Y.; Liu, K.; Jiang, Y.; Huang, L.; et al. Docetaxel-Loaded Nanoparticles of Dendritic Amphiphilic Block Copolymer H40-PLA-b-TPGS for Cancer Treatment. Part. Part. Syst. Charact. 2014, 32, 112–122. [Google Scholar] [CrossRef]
- Xu, W.; Siddiqui, I.A.; Nihal, M.; Pilla, S.; Rosenthal, K.; Mukhtar, H.; Gong, S. Aptamer-conjugated and doxorubicin-loaded unimolecular micelles for targeted therapy of prostate cancer. Biomaterials 2013, 34, 5244–5253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, Y.; Hong, H.; Javadi, A.; Engle, J.W.; Xu, W.; Yang, Y.; Zhang, Y.; Barnhart, T.E.; Cai, W.; Gong, S. Multifunctional unimolecular micelles for cancer-targeted drug delivery and positron emission tomography imaging. Biomaterials 2012, 33, 3071–3082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Xu, J.B.; Chen, H.; Song, Z.F.; Wu, Y.L.; Dai, X.Y.; Kong, J. Acid-Cleavable Unimolecular Micelles from Amphiphilic Star Copolymers for Triggered Release of Anticancer Drugs. Macromol. Biosci. 2017, 17, 1600258. [Google Scholar] [CrossRef] [PubMed]
- Webb, B.A.; Chimenti, M.; Jacobson, M.P.; Barber, D.L. Dysregulated pH: A perfect storm for cancer progression. Nat. Rev. Cancer 2011, 11, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Abandansari, H.S.; Abuali, M.; Nabid, M.R.; Niknejad, H. Enhance chemotherapy efficacy and minimize anticancer drug side effects by using reversibly pH- and redox-responsive cross-linked unimolecular micelles. Polymer 2017, 116, 16–26. [Google Scholar] [CrossRef]
- Lin, W.; Yao, N.; Li, H.; Hanson, S.; Han, W.; Wang, C.; Zhang, L. Co-Delivery of Imiquimod and Plasmid DNA via an Amphiphilic pH-Responsive Star Polymer that Forms Unimolecular Micelles in Water. Polymers 2016, 8, 397. [Google Scholar] [CrossRef]
- Lin, W.; Zhang, X.; Qian, L.; Yao, N.; Pan, Y.; Zhang, L. Doxorubicin-Loaded Unimolecular Micelle-Stabilized Gold Nanoparticles as a Theranostic Nanoplatform for Tumor-Targeted Chemotherapy and Computed Tomography Imaging. Biomacromolecules 2017, 18, 3869–3880. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.X.; Hou, M.L.; Bai, S.; Ma, X.Q.; Gao, Y.E.; Xiao, B.; Xue, P.; Kang, Y.J.; Xu, Z.G.; Li, C.M. Acid-Activatable Theranostic Unimolecular Micelles Composed of Amphiphilic Star-like Polymeric Prodrug with High Drug Loading for Enhanced Cancer Therapy. Mol. Pharm. 2017, 14, 4032–4041. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.; Huang, S.; Yang, C.; Wang, M. Unimolecular Micelles of Amphiphilic Cyclodextrin-Core Star-Like Copolymers with Covalent pH-Responsive Linkage of Anticancer Prodrugs. Mol. Pharm. 2017, 14, 2529–2537. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.; Izzo, L. Dendrimer biocompatibility and toxicity. Adv. Drug Deliv. Rev. 2005, 57, 2215–2237. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, L.; Li, J.; Yang, B.; Wang, C.; Fang, W.; Ji, F.; Wen, Y.; Yao, F. Stable and pH-responsive polyamidoamine based unimolecular micelles capped with a zwitterionic polymer shell for anticancer drug delivery. RSC Adv. 2016, 6, 17728–17739. [Google Scholar] [CrossRef]
- Brinkman, A.M.; Chen, G.; Wang, Y.; Hedman, C.J.; Sherer, N.M.; Havighurst, T.C.; Gong, S.; Xu, W. Aminoflavone-loaded EGFR-targeted unimolecular micelle nanoparticles exhibit anti-cancer effects in triple negative breast cancer. Biomaterials 2016, 101, 20–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaskula-Sztul, R.; Chen, G.; Dammalapati, A.; Harrison, A.; Tang, W.; Gong, S.; Chen, H. AB3-loaded and tumor-targeted unimolecular micelles for medullary thyroid cancer treatment. J. Mater. Chem. B 2017, 5, 151–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.; Chen, G.; Li, J.; Fu, Y.; Mavlyutov, T.A.; Yao, A.; Nickells, R.W.; Gong, S.; Guo, L.-W. An intraocular drug delivery system using targeted nanocarriers attenuates retinal ganglion cell degeneration. J. Control. Release 2017, 247, 153–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.; Shi, X.; Wang, B.; Xie, R.; Guo, L.-W.; Gong, S.; Kent, K.C. Unimolecular Micelle-Based Hybrid System for Perivascular Drug Delivery Produces Long-Term Efficacy for Neointima Attenuation in Rats. Biomacromolecules 2017, 18, 2205–2213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, G.; Gao, H.; Zuo, Y.; Zeng, X.; Tao, W.; Tsai, H.-I.; Mei, L. DACHPt-Loaded Unimolecular Micelles Based on Hydrophilic Dendritic Block Copolymers for Enhanced Therapy of Lung Cancer. ACS Appl. Mater. Interfaces 2017, 9, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Huang, S.; Wang, X.; Wang, M. Theranostic unimolecular micelles of highly fluorescent conjugated polymer bottlebrushes for far red/near infrared bioimaging and efficient anticancer drug delivery. Polym. Chem. 2016, 7, 7455–7468. [Google Scholar] [CrossRef]
- Sun, M.; Yin, W.; Dong, X.; Yang, W.; Zhao, Y.; Yin, M. Fluorescent supramolecular micelles for imaging-guided cancer therapy. Nanoscale 2016, 8, 5302–5312. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Guo, X.; Qu, Q.; Tang, Z.; Wang, Y.; Zhou, S. Actively targeted delivery of anticancer drug to tumor cells by redox-responsive star-shaped micelles. Biomaterials 2014, 35, 8711–8722. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Hong, H.; Chen, G.; Shi, S.; Nayak, T.R.; Theuer, C.P.; Barnhart, T.E.; Cai, W.; Gong, S. Theranostic Unimolecular Micelles Based on Brush-Shaped Amphiphilic Block Copolymers for Tumor-Targeted Drug Delivery and Positron Emission Tomography Imaging. ACS Appl. Mater. Interfaces 2014, 6, 21769–21779. [Google Scholar] [CrossRef] [PubMed]








| Polymer System | Polymer Architecture | Drug Encapsulated | Applications | In Vivo Tests | Reference |
|---|---|---|---|---|---|
| H40-PCL-b-P(OEGMA-Gd-FA) | Multi-arm star block copolymer | Paclitaxel | Tumor therapy & MRI contrast agent | MR imaging in rats | [70] |
| H40-PLA-PEG-OCT | Multi-arm block copolymer | Thailandepsin-A | Neuroendocrine cancer therapy | Antitumor efficacy in mice | [71] |
| H40-PLA-b-TPGS | Multi-arm block copolymer | Docetaxel | Antitumor effect of drug-loaded nanoparticles | Antitumor activity in mice | [72] |
| H40-PLA-PEG-Apt | Aptamer-conjugated multi-arm star block copolymer | Doxorubicin | Targeted therapy for prostate cancer | Higher level of DOX found in mice tumor tissue | [73] |
| H40-P(LG-Hyd-DOX)-b-PEG-OCH3/cRGD/NOTA | Multi-arm block copolymer conjugated with cRGD and macrocyclic chelator | Conjugated Doxorubicin | Cancer-targeted drug delivery and positron emission tomography imaging | Higher level of tumor accumulation in mice | [74] |
| β-CD-(PCL-PAEMA-PPEGMA)21 | 21-arm star-like triblock polymer | Doxorubicin | Tumor therapy & (CT) imaging | Antitumor efficacy in mice | [79] |
| PAMAM-PLA-PEG-OCH3/Cy5.5/GE11 | Multi-arm star block copolymer | Aminoflavone | Triple negative breast cancer therapy | Antitumor efficacy in mice | [84] |
| PAMAM–PVL–PEG–OCH3/Cy5/KE108 | Multi-arm star block copolymer | AB3 | Medullary thyroid cancer therapy | Anticancer efficacy in mice | [85] |
| PAMAM–PVL–PEG–OCH3/Cy5/KE108 | Multi-arm star block copolymer | Thailandepsin-A | Neuroendocrine cancer therapy | Antitumor efficacy in mice | [28] |
| PAMAM–PVL–PEG–Cy5.5/CTB | Multi-arm block copolymer | Dehydroepiandrosterone (DHEA) | Therapy for loss of retinal ganglion cells (glaucoma) | Inhibitory effects on RGC layer degeneration in mice | [86] |
| PAMAM–PVL–PEG | Multi-arm block copolymer | Rapamycin | Preventing neointima-caused (re)stenosis after open surgery | Inhibitory effect on intimal hyperplasia in rats | [87] |
| PAM-PGlub-PEG | Multi-arm block copolymer | 1,2-diaminocyclohexane-platinum(II) | Lung cancer therapy | PK & Antitumor efficacy in mice | [88] |
| PDI-star-(PLA-b-PEEP)8 | Core star block copolymer | Camptothecin | Fluorescence-guided cancer therapy | Tumor growth-inhibitory effect in mice | [90] |
| star-PECLss-FA | Redox responsive-four-arm block copolymer | Doxorubicin | Targeted anticancer drug delivery | Antitumor effect in mice | [91] |
| PHEMA-PLLA-PEG-TRC105 | Brush-shaped block copolymer | Doxorubicin | pH-controlled targeted drug delivery and PET | Tumor uptake in mice | [92] |
| PEI-C18-HPG and HPG-C10-PEG | Derivatized hyperbranched polyglycerols | Paclitaxel | Intravesical bladder cancer therapy | Tumor growth inhibition in mice | [58] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ordanini, S.; Cellesi, F. Complex Polymeric Architectures Self-Assembling in Unimolecular Micelles: Preparation, Characterization and Drug Nanoencapsulation. Pharmaceutics 2018, 10, 209. https://doi.org/10.3390/pharmaceutics10040209
Ordanini S, Cellesi F. Complex Polymeric Architectures Self-Assembling in Unimolecular Micelles: Preparation, Characterization and Drug Nanoencapsulation. Pharmaceutics. 2018; 10(4):209. https://doi.org/10.3390/pharmaceutics10040209
Chicago/Turabian StyleOrdanini, Stefania, and Francesco Cellesi. 2018. "Complex Polymeric Architectures Self-Assembling in Unimolecular Micelles: Preparation, Characterization and Drug Nanoencapsulation" Pharmaceutics 10, no. 4: 209. https://doi.org/10.3390/pharmaceutics10040209