Pharmacokinetic Drug-Drug Interaction and Responsible Mechanism between Memantine and Cimetidine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. LC-MS/MS Analysis of Memantine
2.4. Method Validation
2.4.1. Selectivity
2.4.2. Linearity of Standard Curves
2.4.3. Precisions and Accuracy
2.4.4. Matrix Effect and Recovery
2.4.5. Stability
2.5. Pharmacokinetic (PK) Interaction Study
2.6. Intestinal Permeability Test
2.7. Microsomal Stability
2.8. Data Analysis and Statistics
3. Results
3.1. Validation of Analytical Method
3.1.1. Selectivity
3.1.2. Precision and Accuracy
3.1.3. Matrix Effect and Recovery
3.1.4. Stability
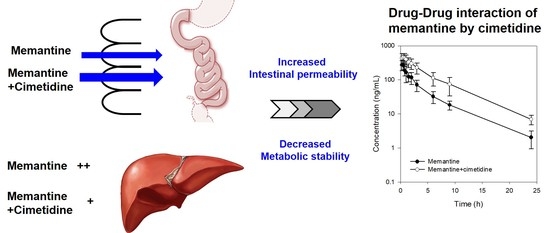
3.2. PK Interaction of Memantine and Cimetidine in Rats
3.3. Effect of Cimetidine on the Intestinal Permeability of Memantine
3.4. Effect of Cimetidine on the Metabolic Stability of Memantine
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Jahn, H. Memory loss in Alzheimer’s disease. Dialogues Clin. Neurosci. 2013, 15, 445–454. [Google Scholar] [PubMed]
- Greig, N.H.; Lahiri, D.K. Advances in understanding alzheimer’s disease, and the contributions of current alzheimer research: Ten years on and beyond. Curr. Alzheimer Res. 2014, 11, 107–109. [Google Scholar] [CrossRef] [PubMed]
- Beconi, M.G.; Howland, D.; Park, L.; Lyons, K.; Giuliano, J.; Dominguez, C.; Munoz-Sanjuan, I.; Pacifici, R. Pharmacokinetics of memantine in rats and mice. PLoS Curr. 2011, 3. [Google Scholar] [CrossRef] [PubMed]
- Molinuevo, J.L.; Llado, A.; Rami, L. Memantine: Targeting glutamate excitotoxicity in alzheimer’s disease and other dementias. Am. J. Alzheimer’s Dis. Other Dement. 2005, 20, 77–85. [Google Scholar] [CrossRef] [PubMed]
- McShane, R.; Areosa Sastre, A.; Minakaran, N. Memantine for dementia. Cochrane Database Syst. Rev. 2006. [Google Scholar] [CrossRef] [PubMed]
- Sani, G.; Serra, G.; Kotzalidis, G.D.; Romano, S.; Tamorri, S.M.; Manfredi, G.; Caloro, M.; Telesforo, C.L.; Caltagirone, S.S.; Panaccione, I.; et al. The role of memantine in the treatment of psychiatric disorders other than the dementias: A review of current preclinical and clinical evidence. CNS Drugs 2012, 26, 663–690. [Google Scholar] [CrossRef] [PubMed]
- Bhateria, M.; Ramakrishna, R.; Pakala, D.B.; Bhatta, R.S. Development of an lc-ms/ms method for simultaneous determination of memantine and donepezil in rat plasma and its application to pharmacokinetic study. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 1001, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Periclou, A.P.; Ventura, D.; Sherman, T.; Rao, N.; Abramowitz, W.T. Lack of pharmacokinetic or pharmacodynamic interaction between memantine and donepezil. Ann. Pharmacother. 2004, 38, 1389–1394. [Google Scholar] [CrossRef] [PubMed]
- Figiel, G.S.; Koumaras, B.; Meng, X.; Strigas, J.; Gunay, I. Long-term safety and tolerability of rivastigmine in patients with alzheimer’s disease switched from donepezil: An open-label extension study. Prim. Care Companion J. Clin. Psychiatry 2008, 10, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Raoufinia, A.; Gold, M.; Nye, J.S.; Ramael, S.; Padmanabhan, M.; Walschap, Y.; Verhaeghe, T.; Zhao, Q. Steady-state pharmacokinetics of galantamine are not affected by addition of memantine in healthy subjects. J. Clin. Pharmacol. 2005, 45, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Lostia, A.M.; Mazzarini, L.; Pacchiarotti, I.; Lionetto, L.; De Rossi, P.; Sanna, L.; Sani, G.; Kotzalidis, G.D.; Girardi, P.; Simmaco, M.; et al. Serum levels of risperidone and its metabolite, 9-hydroxyrisperidone: Correlation between drug concentration and clinical response. Ther. Drug Monit. 2009, 31, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, D. New us fda draft guidance on bioanalytical method validation versus current fda and ema guidelines: Chromatographic methods and isr. Bioanalysis 2014, 6, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Kusuhara, H.; Yokochi, M.; Toyoshima, J.; Inoue, K.; Yuasa, H.; Sugiyama, Y. Competitive inhibition of the luminal efflux by multidrug and toxin extrusions, but not basolateral uptake by organic cation transporter 2, is the likely mechanism underlying the pharmacokinetic drug-drug interactions caused by cimetidine in the kidney. J. Pharmacol. Exp. Ther. 2012, 340, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, S.; Maeda, K.; Inoue, K.; Ohta, K.Y.; Yuasa, H.; Kondo, T.; Nakayama, H.; Horita, S.; Kusuhara, H.; Sugiyama, Y. The inhibition of human multidrug and toxin extrusion 1 is involved in the drug-drug interaction caused by cimetidine. Drug Metab. Dispos. Boil. Fate Chem. 2009, 37, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.; Perlstein, I.; Habib, G.; Pinto, E.; Gilhar, D. The effect of cimetidine on the pharmacodynamics of theophylline-induced seizures and ethanol hypnotic activity. Pharmacol. Toxicol. 1999, 85, 130–132. [Google Scholar] [CrossRef] [PubMed]
- Rafi, J.A.; Frazier, L.M.; Driscoll-Bannister, S.M.; O’Hara, K.A.; Garnett, W.R.; Pugh, C.B. Effect of over-the-counter cimetidine on phenytoin concentrations in patients with seizures. Ann. Pharmacother. 1999, 33, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Kurata, T.; Muraki, Y.; Mizutani, H.; Iwamoto, T.; Okuda, M. Elevated systemic elimination of cimetidine in rats with acute biliary obstruction: The role of renal organic cation transporter OCT2. Drug Metab. Pharmacokinet. 2010, 25, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Piyapolrungroj, N.; Zhou, Y.S.; Li, C.; Liu, G.; Zimmermann, E.; Fleisher, D. Cimetidine absorption and elimination in rat small intestine. Drug Metab. Dispos. Boil. Fate Chem. 2000, 28, 65–72. [Google Scholar]
- Guo, P.; Dong, L.; Yan, W.; Wei, J.; Wang, C.; Zhang, Z. Simultaneous determination of linarin, naringenin and formononetin in rat plasma by lc-ms/ms and its application to a pharmacokinetic study after oral administration of bushen guchi pill. Biomed. Chromatogr. 2015, 29, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Zhang, L.; Chai, C.; Qian, X.C.; Li, W.; Li, J.S.; Di, L.Q.; Cai, B.C. Effects of food and gender on the pharmacokinetics of ginkgolides a, b, c and bilobalide in rats after oral dosing with ginkgo terpene lactones extract. J. Pharm. Biomed. Anal. 2014, 100, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.; Ji, H.K.; Goo, S.H.; Nam, S.J.; Kang, Y.J.; Lee, E.; Liu, K.H.; Choi, M.K.; Song, I.S. Involvement of intestinal efflux and metabolic instability in the pharmacokinetics of platycodin d in rats. Drug Metab. Pharmacokinet. 2017, 32, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Levin, V.A. Relationship of octanol/water partition coefficient and molecular weight to rat brain capillary permeability. J. Med. Chem. 1980, 23, 682–684. [Google Scholar] [CrossRef] [PubMed]
- Stewart, B.H.; Chan, O.H.; Lu, R.H.; Reyner, E.L.; Schmid, H.L.; Hamilton, H.W.; Steinbaugh, B.A.; Taylor, M.D. Comparison of intestinal permeabilities determined in multiple in vitro and in situ models: Relationship to absorption in humans. Pharm. Res. 1995, 12, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.K.; Lee, J.; Nam, S.J.; Kang, Y.J.; Han, Y.; Choi, K.; Choi, Y.A.; Kwon, M.; Lee, D.; Song, I.S. Pharmacokinetics of jaspine b and enhancement of intestinal absorption of jaspine b in the presence of bile acid in rats. Mar. Drugs 2017, 15, 279. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, S.H.; Noh, Y.H.; Choi, B.M.; Noh, G.J.; Park, W.D.; Kim, E.J.; Cho, I.H.; Bae, C.S. Pharmacokinetics of memantine after a single and multiple dose of oral and patch administration in rats. Basic Clin. Pharmacol. Toxicol. 2016, 118, 122–127. [Google Scholar] [CrossRef] [PubMed]
- McConnell, E.L.; Basit, A.W.; Murdan, S. Measurements of rat and mouse gastrointestinal ph, fluid and lymphoid tissue, and implications for in-vivo experiments. J. Pharm. Pharmacol. 2008, 60, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.W.; Xia, L.; Gong, L.M.; Ruan, L.M.; Zhao, Y.G. Rapid determination of memantine in human plasma by using nanoring carboxyl-functionalized paramagnetic molecularly imprinted polymer d-mu-spe and uflc-ms/ms. Drug Test. Anal. 2015, 7, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Suresh, P.S.; Mullangi, R.; Sukumaran, S.K. Highly sensitive lc-ms/ms method for determination of galantamine in rat plasma: Application to pharmacokinetic studies in rats. Biomed. Chromatogr. 2014, 28, 1633–1640. [Google Scholar] [CrossRef] [PubMed]
- Noetzli, M.; Ansermot, N.; Dobrinas, M.; Eap, C.B. Simultaneous determination of antidementia drugs in human plasma: Procedure transfer from hplc-ms to uplc-ms/ms. J. Pharm. Biomed. Anal. 2012, 64–65, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Konda, R.K.; Challa, B.R.; Chandu, B.R.; Chandrasekhar, K.B. Bioanalytical method development and validation of memantine in human plasma by high performance liquid chromatography with tandem mass spectrometry: Application to bioequivalence study. J. Anal. Methods Chem. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.A.; Campos, D.R.; Bernasconi, G.; Calafatti, S.; Barros, F.A.; Eberlin, M.N.; Meurer, E.C.; Paris, E.G.; Pedrazzoli, J. Determination of memantine in human plasma by liquid chromatography-electrospray tandem mass spectrometry: Application to a bioequivalence study. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 848, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Mehta, D.C.; Short, J.L.; Nicolazzo, J.A. Memantine transport across the mouse blood-brain barrier is mediated by a cationic influx h+ antiporter. Mol. Pharm. 2013, 10, 4491–4498. [Google Scholar] [CrossRef] [PubMed]
- Yabuuchi, H.; Tamai, I.; Nezu, J.; Sakamoto, K.; Oku, A.; Shimane, M.; Sai, Y.; Tsuji, A. Novel membrane transporter octn1 mediates multispecific, bidirectional, and ph-dependent transport of organic cations. J. Pharmacol. Exp. Ther. 1999, 289, 768–773. [Google Scholar] [PubMed]
- Levine, M.; Law, E.Y.; Bandiera, S.M.; Chang, T.K.; Bellward, G.D. In vivo cimetidine inhibits hepatic CYP2C6 and CYP2C11 but not cyp1a1 in adult male rats. J. Pharmacol. Exp. Ther. 1998, 284, 493–499. [Google Scholar] [PubMed]
- Muller, F.; Weitz, D.; Derdau, V.; Sandvoss, M.; Mertsch, K.; Konig, J.; Fromm, M.F. Contribution of mate1 to renal secretion of the nmda receptor antagonist memantine. Mol. Pharm. 2017, 14, 2991–2998. [Google Scholar] [CrossRef] [PubMed]




| Memantine | Spiked (ng/mL) | Inter-day | Intra-day | ||||
|---|---|---|---|---|---|---|---|
| Measured (ng/mL) | Accuracy (%) | Precision (%) | Measured (ng/mL) | Accuracy (%) | Precision (%) | ||
| Low QC | 0.6 | 0.62 ± 0.05 | 101.2 | 8.10 | 0.68 ± 0.10 | 113.8 | 14.1 |
| Medium QC | 20 | 20.6 ± 2.13 | 108.6 | 10.3 | 21.98 ± 0.37 | 109.9 | 1.68 |
| High QC | 500 | 497.6 ± 56.9 | 104.2 | 11.4 | 555.6 ± 23.5 | 111.2 | 4.23 |
| Compounds | Spiked (ng/mL) | Recovery (%) | Matrix Effect (%) | |
|---|---|---|---|---|
| Propranolol | IS | 20 | 87.7 ± 1.96 (2.2) | 102.9 ± 1.20 (1.2) |
| Memantine | Low QC | 0.6 | 90.7 ± 3.11 (3.4) | 108.2 ± 13.7 (12.6) |
| Medium QC | 20 | 91.2 ± 3.38 (3.7) | 92.7 ± 5.43 (5.9) | |
| High QC | 500 | 87.5 ± 3.18 (3.6) | 91.1 ± 7.55 (8.3) | |
| Spiked (ng/mL) | Measured (ng/mL) | Accuracy (%) | Precision (%) |
|---|---|---|---|
| Bench-top stability for 12 h in plasma | |||
| Low QC (0.6) | 0.67 ± 0.10 | 112.2 | 14.9 |
| High QC (500) | 532.7 ± 27.6 | 106.6 | 5.2 |
| Three freeze-thaw cycles | |||
| Low QC (0.6) | 0.51 ± 0.05 | 85.1 | 10.7 |
| High QC (500) | 539.3 ± 16.4 | 107.9 | 3.1 |
| Post treatment stability for 24 h | |||
| Low QC (0.6) | 0.59 ± 0.04 | 98.4 | 7.6 |
| High QC (500) | 540.1 ± 13.7 | 108 | 2.5 |
| PK Parameters | IV | PO | |||
|---|---|---|---|---|---|
| Memantine (3 mg/kg) | Memantine (3 mg/kg) + Cimetidine (10 mg/kg) | Memantine (5 mg/kg) | Memantine (5 mg/kg) + Cimetidine (50 mg/kg) | ||
| Cmax | μg/mL | 686.84 ± 100.75 | 759.04 ± 240.18 | 293.45 ± 90.90 | 486.78 ± 140.75 * |
| Tmax | h | 0.35 ± 0.14 | 0.50 ± 0.18 | ||
| AUC24h | μg·h/mL | 1089.58 ± 116.55 | 1724.52 ± 241.36 * | 811.38 ± 238.01 | 2392.69 ± 950.06 * |
| AUC∞ | μg·h/mL | 1121.98 ± 116.74 | 1765.56 ± 237.78 * | 825.60 ± 241.92 | 2439.28 ± 951.72 * |
| t1/2 | h | 5.06 ± 0.38 | 4.98 ± 0.59 | 4.51 ± 0.92 | 4.70 ± 0.75 |
| Vd,ss | L/kg | 19.70 ± 3.32 | 12.50 ± 3.16 | ||
| CL | mL/min/kg | 44.87 ± 4.40 | 28.67 ± 3.92 * | ||
| CL/F | mL/min/kg | 107.63 ± 28.83 | 39.06 ± 16.00 * | ||
| CLrenal | mL/min/kg | 12.88 ± 3.89 | 10.64 ± 5.73 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.A.; Song, I.-S.; Choi, M.-K. Pharmacokinetic Drug-Drug Interaction and Responsible Mechanism between Memantine and Cimetidine. Pharmaceutics 2018, 10, 119. https://doi.org/10.3390/pharmaceutics10030119
Choi YA, Song I-S, Choi M-K. Pharmacokinetic Drug-Drug Interaction and Responsible Mechanism between Memantine and Cimetidine. Pharmaceutics. 2018; 10(3):119. https://doi.org/10.3390/pharmaceutics10030119
Chicago/Turabian StyleChoi, Young A., Im-Sook Song, and Min-Koo Choi. 2018. "Pharmacokinetic Drug-Drug Interaction and Responsible Mechanism between Memantine and Cimetidine" Pharmaceutics 10, no. 3: 119. https://doi.org/10.3390/pharmaceutics10030119