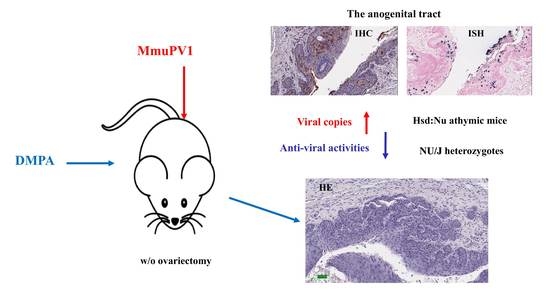
Depo Medroxyprogesterone (DMPA) Promotes Papillomavirus Infections but Does Not Accelerate Disease Progression in the Anogenital Tract of a Mouse Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice and MmuPV1 Infection
2.2. Vaginal and Anal Lavage for DNA Extraction and qPCR Analysis
2.3. RNA Detection for Virus by qRT-PCR Analysis
2.4. Cytokine Profiling
2.5. Immunohistochemistry and In Situ Hybridization Analyses of Infected Tissues
2.6. Histology and Interpretation
2.7. Statistical Analysis
3. Results
3.1. DMPA Treatment Increased Viral Susceptibility at the Lower Genital and Anal Tract of Athymic Nude Mice
3.2. DMPA Treatment Increased Viral Susceptibility and Persistence at the Lower Genital Tract of NU/J Heterozygous (Foxn1nu/+) Mice
3.3. DMPA Treatment Failed to Increase Viral Susceptibility in Ovariectomized Heterozygous NU/J Mice
3.4. DMPA Treatment Dysregulated Anti-Viral Activities at the Lower Genital Tissues
3.5. Long-Term Contraceptive Treatment Did Not Promote More Advanced Diseases in Hsd:Nu Athymic Mice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, H.J. Current status and future prospects for human papillomavirus vaccines. Arch. Pharm. Res. 2017, 40, 1050–1063. [Google Scholar] [CrossRef] [PubMed]
- Roden, R.B.S.; Stern, P.L. Opportunities and challenges for human papillomavirus vaccination in cancer. Nat. Rev. Cancer 2018, 18, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Blake, D.R.; Middleman, A.B. Human Papillomavirus Vaccine Update. Pediatr. Clin. N. Am. 2017, 64, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Razzaghi, H.; Saraiya, M.; Thompson, T.D.; Henley, S.J.; Viens, L.; Wilson, R. Five-year relative survival for human papillomavirus-associated cancer sites. Cancer 2018, 124, 203–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serrano, B.; Brotons, M.; Bosch, F.X.; Bruni, L. Epidemiology and burden of HPV-related disease. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 47, 14–26. [Google Scholar] [CrossRef]
- Liu, S.H.; Brotman, R.M.; Zenilman, J.M.; Gravitt, P.E.; Cummings, D.A. Menstrual cycle and detectable human papillomavirus in reproductive-age women: A time series study. J. Infect. Dis. 2013, 208, 1404–1415. [Google Scholar] [CrossRef] [Green Version]
- Hall, O.J.; Klein, S.L. Progesterone-based compounds affect immune responses and susceptibility to infections at diverse mucosal sites. Mucosal. Immunol. 2017, 10, 1097–1107. [Google Scholar] [CrossRef] [Green Version]
- Herrero, R.; Brinton, L.A.; Reeves, W.C.; Brenes, M.M.; Tenorio, F.; de Britton, R.C.; Gaitan, E.; Montalvan, P.; Garcia, M.; Rawls, W.E. Risk factors for invasive carcinoma of the uterine cervix in Latin America. Bull. Pan Am. Health Organ. 1990, 24, 263–283. [Google Scholar]
- Bright, P.L.; Norris, T.A.; Morrison, C.S.; Wong, E.L.; Kwok, C.; Yacobson, I.; Royce, R.A.; Tucker, H.O.; Blumenthal, P.D. Hormonal contraception and area of cervical ectopy: A longitudinal assessment. Contraception 2011, 84, 512–519. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.J.; Palefsky, J.M. HPV-Associated Anal Cancer in the HIV/AIDS Patient. Cancer Treat. Res. 2019, 177, 183–209. [Google Scholar] [PubMed]
- Liszewski, W.; Ananth, A.T.; Ploch, L.E.; Rogers, N.E. Anal Pap smears and anal cancer: What dermatologists should know. J. Am. Acad. Dermatol. 2014, 71, 985–992. [Google Scholar] [CrossRef]
- Gaisa, M.; Sigel, K.; Hand, J.; Goldstone, S. High rates of anal dysplasia in HIV-infected men who have sex with men, women, and heterosexual men. AIDS 2014, 28, 215–222. [Google Scholar] [CrossRef]
- Benevolo, M.; Dona, M.G.; Ravenda, P.S.; Chiocca, S. Anal human papillomavirus infection: Prevalence, diagnosis and treatment of related lesions. Expert Rev. Anti. Infect. Ther. 2016, 14, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Christensen, N.D.; Budgeon, L.R.; Cladel, N.M.; Hu, J. Recent advances in preclinical model systems for papillomaviruses. Virus Res. 2017, 231, 108–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doorbar, J. Model systems of human papillomavirus-associated disease. J. Pathol. 2016, 238, 166–179. [Google Scholar] [CrossRef] [Green Version]
- Cladel, N.M.; Budgeon, L.R.; Cooper, T.K.; Balogh, K.K.; Christensen, N.D.; Myers, R.; Majerciak, V.; Gotte, D.; Zheng, Z.M.; Hu, J. Mouse papillomavirus infections spread to cutaneous sites with progression to malignancy. J. Gen. Virol. 2017, 98, 2520–2529. [Google Scholar] [CrossRef]
- Cladel, N.M.; Budgeon, L.R.; Balogh, K.K.; Cooper, T.K.; Hu, J.; Christensen, N.D. A novel pre-clinical murine model to study the life cycle and progression of cervical and anal papillomavirus infections. PLoS ONE 2015, 10, e0120128. [Google Scholar] [CrossRef] [Green Version]
- Cladel, N.M.; Budgeon, L.R.; Balogh, K.K.; Cooper, T.K.; Hu, J.; Christensen, N.D. Mouse papillomavirus MmuPV1 infects oral mucosa and preferentially targets the base of the tongue. Virology 2016, 488, 73–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.; Budgeon, L.R.; Cladel, N.M.; Balogh, K.; Myers, R.; Cooper, T.K.; Christensen, N.D. Tracking vaginal, anal and oral infection in a mouse papillomavirus infection model. J. Gen. Virol. 2015, 96, 3554–3565. [Google Scholar] [CrossRef] [PubMed]
- Spurgeon, M.E.; Uberoi, A.; McGregor, S.M.; Wei, T.; Ward-Shaw, E.; Lambert, P.F. A Novel In Vivo Infection Model To Study Papillomavirus-Mediated Disease of the Female Reproductive Tract. mBio 2019, 10, e00180-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spurgeon, M.E.; Lambert, P.F. Sexual transmission of murine papillomavirus (MmuPV1) in Mus musculus. eLife 2019, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Uberoi, A.; Lambert, P.F. Rodent Papillomaviruses. Viruses 2017, 9, 362. [Google Scholar] [CrossRef] [Green Version]
- Miller, L.; Patton, D.L.; Meier, A.; Thwin, S.S.; Hooton, T.M.; Eschenbach, D.A. Depomedroxyprogesterone-induced hypoestrogenism and changes in vaginal flora and epithelium. Obstet. Gynecol. 2000, 96, 431–439. [Google Scholar]
- Kleynhans, L.; Du, P.N.; Allie, N.; Jacobs, M.; Kidd, M.; Van Helden, P.D.; Walzl, G.; Ronacher, K. The contraceptive depot medroxyprogesterone acetate impairs mycobacterial control and inhibits cytokine secretion in mice infected with Mycobacterium tuberculosis. Infect. Immun. 2013, 81, 1234–1244. [Google Scholar] [CrossRef] [Green Version]
- Chung, S.H.; Shin, M.K.; Korach, K.S.; Lambert, P.F. Requirement for stromal estrogen receptor alpha in cervical neoplasia. Horm. Cancer 2013, 4, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Marks, M.; Gravitt, P.E.; Gupta, S.B.; Liaw, K.L.; Kim, E.; Tadesse, A.; Phongnarisorn, C.; Wootipoom, V.; Yuenyao, P.; Vipupinyo, C.; et al. The association of hormonal contraceptive use and HPV prevalence. Int. J. Cancer 2011, 128, 2962–2970. [Google Scholar] [CrossRef] [PubMed]
- Marks, M.; Gravitt, P.E.; Gupta, S.B.; Liaw, K.L.; Tadesse, A.; Kim, E.; Phongnarisorn, C.; Wootipoom, V.; Yuenyao, P.; Vipupinyo, C.; et al. Combined oral contraceptive use increases HPV persistence but not new HPV detection in a cohort of women from Thailand. J. Infect. Dis. 2011, 204, 1505–1513. [Google Scholar] [CrossRef] [Green Version]
- Tang, W.W.; Young, M.P.; Mamidi, A.; Regla-Nava, J.A.; Kim, K.; Shresta, S. A Mouse Model of Zika Virus Sexual Transmission and Vaginal Viral Replication. Cell Rep. 2016, 17, 3091–3098. [Google Scholar] [CrossRef] [Green Version]
- Martin, H.L., Jr.; Nyange, P.M.; Richardson, B.A.; Lavreys, L.; Mandaliya, K.; Jackson, D.J.; Ndinya-Achola, J.O.; Kreiss, J. Hormonal contraception, sexually transmitted diseases, and risk of heterosexual transmission of human immunodeficiency virus type 1. J. Infect. Dis. 1998, 178, 1053–1059. [Google Scholar] [CrossRef]
- Roberts, J.N.; Buck, C.B.; Thompson, C.D.; Kines, R.; Bernardo, M.; Choyke, P.L.; Lowy, D.R.; Schiller, J.T. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat. Med. 2007, 13, 857–861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cladel, N.M.; Budgeon, L.R.; Balogh, K.K.; Cooper, T.K.; Brendle, S.A.; Christensen, N.D.; Schell, T.D.; Hu, J. Mouse papillomavirus infection persists in mucosal tissues of an immunocompetent mouse strain and progresses to cancer. Sci. Rep. 2017, 7, 16932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taiyeb, A.M.; Ridha, M.T.; Sayes, C.M.; Dees, W.L.; Kraemer, D.C. Cilostazol administered to female mice induces ovulation of immature oocytes: A contraceptive animal model. Life Sci. 2014, 96, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Albarzanchi, A.M.; Sayes, C.M.; Ridha Albarzanchi, M.T.; Fajt, V.R.; Dees, W.L.; Kraemer, D.C. Cilostazol blocks pregnancy in naturally cycling mice. Contraception 2013, 87, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Beziat, V.; Rapaport, F.; Hu, J.; Titeux, M.; Bonnet des Claustres, M.; Bourgey, M.; Griffin, H.; Bandet, E.; Ma, C.S.; Sherkat, R.; et al. Humans with inherited T cell CD28 deficiency are susceptible to skin papillomaviruses but are otherwise healthy. Cell 2021, 184, 3812–3828. [Google Scholar] [CrossRef] [PubMed]
- Cladel, N.M.; Budgeon, L.R.; Cooper, T.K.; Balogh, K.K.; Hu, J.; Christensen, N.D. Secondary infections, expanded tissue tropism, and evidence for malignant potential in immunocompromised mice infected with Mus musculus papillomavirus 1 DNA and virus. J. Virol. 2013, 87, 9391–9395. [Google Scholar] [CrossRef] [Green Version]
- Cladel, N.M.; Jiang, P.; Li, J.J.; Peng, X.; Cooper, T.K.; Majerciak, V.; Balogh, K.K.; Meyer, T.J.; Brendle, S.A.; Budgeon, L.R.; et al. Papillomavirus can be transmitted through the blood and produce infections in blood recipients: Evidence from two animal models. Emerg. Microbes Infect. 2019, 8, 1108–1121. [Google Scholar] [CrossRef] [Green Version]
- Brendle, S.; Li, J.J.; Cladel, N.M.; Shearer, D.A.; Budgeon, L.R.; Balogh, K.K.; Atkins, H.; Costa-Fujishima, M.; Lopez, P.; Christensen, N.D.; et al. Mouse Papillomavirus L1 and L2 Are Dispensable for Viral Infection and Persistence at Both Cutaneous and Mucosal Tissues. Viruses 2021, 13, 1824. [Google Scholar] [CrossRef]
- Kaushic, C.; Ashkar, A.A.; Reid, L.A.; Rosenthal, K.L. Progesterone increases susceptibility and decreases immune responses to genital herpes infection. J. Virol. 2003, 77, 4558–4565. [Google Scholar] [CrossRef] [Green Version]
- Gallichan, W.S.; Rosenthal, K.L. Effects of the estrous cycle on local humoral immune responses and protection of intranasally immunized female mice against herpes simplex virus type 2 infection in the genital tract. Virology 1996, 224, 487–497. [Google Scholar] [CrossRef] [Green Version]
- Byers, S.L.; Wiles, M.V.; Dunn, S.L.; Taft, R.A. Mouse estrous cycle identification tool and images. PLoS ONE 2012, 7, e35538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bronson, F.H.; Vom Saal, F.S. Control of the preovulatory release of luteinizing hormone by steroids in the mouse. Endocrinology 1979, 104, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Caligioni, C.S. Assessing reproductive status/stages in mice. Curr. Protoc. Neurosci. 2009, 48, A-4I. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodworth, C.D.; McMullin, E.; Iglesias, M.; Plowman, G.D. Interleukin 1a and tumor necrosis factor a stimulate autocrine amphiregulin expression and proliferation of human papillomavirus- immortalized and carcinoma-derived cervical epithelial cells. Proc. Natl. Acad. Sci. USA 1995, 92, 2840–2844. [Google Scholar] [CrossRef] [Green Version]
- Wilson, S.S.; Wiens, M.E.; Smith, J.G. Antiviral mechanisms of human defensins. J. Mol. Biol. 2013, 425, 4965–4980. [Google Scholar] [CrossRef]
- Buck, C.B.; Day, P.M.; Thompson, C.D.; Lubkowski, J.; Lu, W.; Lowy, D.R.; Schiller, J.T. Human alpha-defensins block papillomavirus infection. Proc. Natl. Acad. Sci. USA 2006, 103, 1516–1521. [Google Scholar] [CrossRef] [Green Version]
- La, V.C.; Boccia, S. Oral contraceptives, human papillomavirus and cervical cancer. Eur. J. Cancer Prev. 2014, 23, 110–112. [Google Scholar]
- Cropsey, K.L.; Matthews, C.; Campbel, S.; Ivey, S.; Adawadkar, S. Long-term, reversible contraception use among high-risk women treated in a university-based gynecology clinic: Comparison between IUD and depo-provera. J. Womens Health 2010, 19, 349–353. [Google Scholar] [CrossRef]
- Marais, D.; Carrara, H.; Kay, P.; Ramjee, G.; Allan, B.; Williamson, A.L. The impact of the use of COL-1492, a nonoxynol-9 vaginal gel, on the presence of cervical human papillomavirus in female sex workers. Virus Res. 2006, 121, 220–222. [Google Scholar] [CrossRef]
- De, S.F.; Restaino, S.; De, S.D.; Stabile, G.; Banco, R.; Busetti, M.; Barbati, G.; Guaschino, S. Effects of hormonal contraception on vaginal flora. Contraception 2012, 86, 526–529. [Google Scholar]
- Berry, G.; MacLennan, R.; Shearman, R.; Jelihovsky, T.; Booth, J.C.; Molina, R.; Martinez, L.; Salas, O.; Dabancens, A.; Chen, Z.H. Invasive squamous-cell cervical carcinoma and combined oral contraceptives- results from a multinational study. Int. J. Cancer 1993, 55, 228. [Google Scholar]
- Mostad, S.B.; Kreiss, J.K.; Ryncarz, A.J.; Mandaliya, K.; Chohan, B.; Ndinya-Achola, J.; Bwayo, J.J.; Corey, L. Cervical shedding of herpes simplex virus in human immunodeficiency virus-infected women: Effects of hormonal contraception, pregnancy, and vitamin A deficiency. J. Infect. Dis. 2000, 181, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, T.; Takashima, Y.; Hirayama, S.; Okada, H. Effects of menstrual cycle on gene transfection through mouse vagina for DNA vaccine. Int. J. Pharm. 2008, 360, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Dizzell, S.E.; Leung, V.; Nazli, A.; Zahoor, M.A.; Fichorova, R.N.; Kaushic, C. Effects of Female Sex Hormones on Susceptibility to HSV-2 in Vaginal Cells Grown in Air-Liquid Interface. Viruses 2016, 8, 241. [Google Scholar] [CrossRef] [PubMed]
- Kaushic, C.; Roth, K.L.; Anipindi, V.; Xiu, F. Increased prevalence of sexually transmitted viral infections in women: The role of female sex hormones in regulating susceptibility and immune responses. J. Reprod. Immunol. 2011, 88, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Scagnolari, C.; Cannella, F.; Pierangeli, A.; Mellinger Pilgrim, R.; Antonelli, G.; Rowley, D.; Wong, M.; Best, S.; Xing, D.; Roden, R.B.S.; et al. Insights into the Role of Innate Immunity in Cervicovaginal Papillomavirus Infection from Studies Using Gene-Deficient Mice. J. Virol. 2020, 94, 12. [Google Scholar] [CrossRef] [PubMed]
- Tarin, J.J.; Perez-Albala, S.; Cano, A. Stage of the estrous cycle at the time of pregnant mare’s serum gonadotropin injection affects the quality of ovulated oocytes in the mouse. Mol. Reprod. Dev. 2002, 61, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, T.; Takahashi, K.W. Effect of pregnant mare’s serum gonadotropin on increased ovulation in guinea pigs with synchronized estrous cycle. Exp. Anim. 1989, 38, 81–83. [Google Scholar] [CrossRef] [Green Version]
- Chung, S.H.; Lambert, P.F. Prevention and treatment of cervical cancer in mice using estrogen receptor antagonists. Proc. Natl. Acad. Sci. USA 2009, 106, 19467–19472. [Google Scholar] [CrossRef] [Green Version]
- Hsu, I.; Chuang, K.L.; Slavin, S.; Da, J.; Lim, W.X.; Pang, S.T.; O’Brien, J.H.; Yeh, S. Suppression of ERbeta signaling via ERbeta knockout or antagonist protects against bladder cancer development. Carcinogenesis 2014, 35, 651–661. [Google Scholar] [CrossRef]
- Mhatre, M.; McAndrew, T.; Carpenter, C.; Burk, R.D.; Einstein, M.H.; Herold, B.C. Cervical intraepithelial neoplasia is associated with genital tract mucosal inflammation. Sex Transm. Dis. 2012, 39, 591–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nunes, R.A.L.; Morale, M.G.; Silva, G.A.F.; Villa, L.L.; Termini, L. Innate immunity and HPV: Friends or foes. Clinics 2018, 73 (Suppl. 1), e549s. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Cladel, N.M.; Budgeon, L.R.; Balogh, K.K.; Christensen, N.D. The Mouse Papillomavirus Infection Model. Viruses 2017, 9, 246. [Google Scholar] [CrossRef]
- Hall, O.J.; Nachbagauer, R.; Vermillion, M.S.; Fink, A.L.; Phuong, V.; Krammer, F.; Klein, S.L. Progesterone-Based Contraceptives Reduce Adaptive Immune Responses and Protection against Sequential Influenza A Virus Infections. J. Virol. 2017, 91, e02160-16. [Google Scholar] [CrossRef] [Green Version]
- Smith-McCune, K.K.; Hilton, J.F.; Shanmugasundaram, U.; Critchfield, J.W.; Greenblatt, R.M.; Seidman, D.; Averbach, S.; Giudice, L.C.; Shacklett, B.L. Effects of depot-medroxyprogesterone acetate on the immune microenvironment of the human cervix and endometrium: Implications for HIV susceptibility. Mucosal. Immunol. 2017, 10, 1270–1278. [Google Scholar] [CrossRef] [PubMed]
- Chagas, B.S.; Gurgel, A.; Paiva Junior, S.S.L.; Lima, R.C.P.; Cordeiro, M.N.; Moura, R.R.; Coelho, A.V.C.; Nascimento, K.C.G.; Silva Neto, J.C.; Crovella, S.; et al. Synergic effect of oral contraceptives, GSTP1 polymorphisms, and high-risk HPV infection in development of cervical lesions. Genet. Mol. Res. 2017, 16, 3. [Google Scholar] [CrossRef] [PubMed]
- Tota, J.E.; Ramanakumar, A.V.; Mahmud, S.M.; Trevisan, A.; Villa, L.L.; Franco, E.L. Cervical human papillomavirus detection is not affected by menstrual phase. Sex Transm. Infect. 2013, 89, 202–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Cytokine | Sense Primer | Anti-Sense Primer |
|---|---|---|
| IL6 | 5′-AACGATGATGCACTTGCAGA-3′ | 5′-GGTACTCCAG AAGACCAGAG G-3′ |
| IL36γ | 5′ACCACACCCGGACAGGTGGA-3′ | 5′TGGGGTTGCCAGTCTTGGAGGA-3′ |
| IL-17A | 5′GCTCCAGAAGGCCCTCAGA-3′ | 5′AGCTTTCCCTCCGCATTGA-3′ |
| IL-23p19 | 5′ACGGGGCACATTATTTTTAGTCT-3′ | 5′ ATGCTGGATTGCAGAGCAGTA-3′ |
| GATA-3 | 5′ACCACGGGAGCCAGGTATG -3′ | 5′CGGAGGGTAAACGGACAGAG-3′ |
| TNFa | 5′-AG CCCCCAGTCTGTATCCTT-3′ | 5′-CTCC CTT TGCAGAACTCAGG-3′ |
| ARRb2 | 5′-GGCAAGCGCGACTTTGTAG-3′ | 5′-GTGAGGGTCACGAACACTTTC-3′ |
| LGR5 | 5′-TGCCCATCACACTGTCACTGT-3′ | 5′-CACCCTGAGCAGCATCCTG-3′ |
| GAPDH | 5′-TGGCAAAGTGGAGATTGTTGCC-3′ | 5′-AAGATGGTGATGGGCTTCCCG-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Brendle, S.A.; Li, J.J.; Walter, V.; Cladel, N.M.; Cooper, T.; Shearer, D.A.; Balogh, K.K.; Christensen, N.D. Depo Medroxyprogesterone (DMPA) Promotes Papillomavirus Infections but Does Not Accelerate Disease Progression in the Anogenital Tract of a Mouse Model. Viruses 2022, 14, 980. https://doi.org/10.3390/v14050980
Hu J, Brendle SA, Li JJ, Walter V, Cladel NM, Cooper T, Shearer DA, Balogh KK, Christensen ND. Depo Medroxyprogesterone (DMPA) Promotes Papillomavirus Infections but Does Not Accelerate Disease Progression in the Anogenital Tract of a Mouse Model. Viruses. 2022; 14(5):980. https://doi.org/10.3390/v14050980
Chicago/Turabian StyleHu, Jiafen, Sarah A. Brendle, Jingwei J. Li, Vonn Walter, Nancy M. Cladel, Timothy Cooper, Debra A. Shearer, Karla K. Balogh, and Neil D. Christensen. 2022. "Depo Medroxyprogesterone (DMPA) Promotes Papillomavirus Infections but Does Not Accelerate Disease Progression in the Anogenital Tract of a Mouse Model" Viruses 14, no. 5: 980. https://doi.org/10.3390/v14050980