The Link between Cannabis Use, Immune System, and Viral Infections
Abstract
:1. Introduction
2. Methods
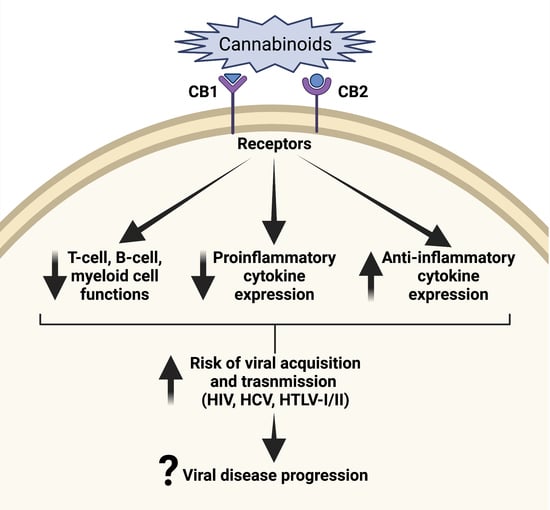
3. Cannabis and Immune System
4. Cannabis and Infections
5. Discussion
6. Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Drug Report 2019. Available online: https://reliefweb.int/sites/reliefweb.int/files/resources/WDR19_Booklet_1_EXECUTIVE_SUMMARY.pdf (accessed on 21 February 2021).
- Key Substance Use and Mental Health Indicators in the United States: Results from the 2019 National Survey on Drug Use and Health (HHS Publication No. PEP20-07-01-001, NSDUH Series H-55). 2020. Available online: https://www.samhsa.gov/data/ (accessed on 21 February 2021).
- Wagner, F.A.; Anthony, J.C. From first drug use to drug dependence; developmental periods of risk for dependence upon marijuana, cocaine, and alcohol. Neuropsychopharmacology 2002, 26, 479–488. [Google Scholar] [CrossRef]
- Johnston, L.D.; Miech, R.A.; O’Malley, P.M.; Bachman, J.G.; Schulenberg, J.E.; Patrick, M.E. Monitoring the Future: Key Findings on Adolescent Drug Use, National Survey Results on Drug Use 1975–2018: Overview, Key Findings on Adolescent Drug Use. 2018. Available online: https://deepblue.lib.umich.edu/bitstream/handle/2027.42/148123/Overview%202018%20FINAL%20print%201-30.pdf?sequence=1&isAllowed=y (accessed on 21 February 2021).
- Centers for Disease Control and Prevention: Global HIV and TB. 2021. Available online: https://www.cdc.gov/globalhivtb/index.html (accessed on 21 February 2021).
- Center for Disease Control and Prevention. Global Viral Hepatitis: Millions of People Are affected. 2021. Available online: https://www.cdc.gov/hepatitis/global/ (accessed on 21 February 2021).
- National Organization for Rare Disorders. Rare Disease Database: HTLV Type I and Type II. 2021. Available online: https://rarediseases.org/rare-diseases/htlv-type-i-and-type-ii/#:~:text=It%20is%20estimated%20that%20between,I%20carriers%20will%20develop%20ATL (accessed on 21 February 2021).
- Johns Hopkins University of Medicine Coronavirus Resource Center. Available online: https://coronavirus.jhu.edu/ (accessed on 23 May 2021).
- Volkow, N.D.; Baler, R.D.; Compton, W.M.; Weiss, S.R. Adverse health effects of marijuana use. N. Engl. J. Med. 2014, 370, 2219–2227. [Google Scholar] [CrossRef] [Green Version]
- Khalsa, J.H. Medical and Health Consequences of Marijuana. In Marijuana and the Cannabinoids; El Sohly, M.A., Ed.; Humana Press, Inc.: Totowa, NJ, USA, 2007; pp. 237–252. [Google Scholar]
- Khalsa, J.; Baler, R. Medical Consequences of Marijuana Use. In Cannabis Use Disorder; Montoya, I., Weiss, S., Eds.; Springer: Cham, Switzerland, 2019; Volume 15, pp. 157–167. [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on the Health Effects of Marijuana: An Evidence Review and Research Agenda. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research; The National Academies Press: Washington, DC, USA, 2017; p. 486. [Google Scholar]
- ElSohly, M.A.; Radwan, M.M.; Gul, W.; Chandra, S.; Galal, A. Phytochemistry of Cannabis sativa L. Prog. Chem. Org. Nat. Prod. 2017, 103, 1–36. [Google Scholar]
- Cabral, G.A.; Rogers, T.J.; Lichtman, A.H. Turning over a New Leaf: Cannabinoid and Endocannabinoid Modulation of Immune Function. J. Neuroimmune Pharmacol. 2015, 10, 193–203. [Google Scholar] [CrossRef] [Green Version]
- Olah, A.; Szekanecz, Z.; Biro, T. Targeting Cannabinoid Signaling in the Immune System: “High”-ly Exciting Questions, Possibilities, and Challenges. Front. Immunol. 2017, 8, 1487. [Google Scholar] [CrossRef] [Green Version]
- Almogi-Hazan, O.; Or, R. Cannabis, the Endocannabinoid System and Immunity—The Journey from the Bedside to the Bench and Back. Int. J. Mol. Sci. 2020, 21, 4448. [Google Scholar] [CrossRef]
- Da Silva, T.; Hafizi, S.; Watts, J.J.; Weickert, C.S.; Meyer, J.H.; Houle, S.; Rusjan, P.; Mizrahi, R. In Vivo Imaging of Translocator Protein in Long-term Cannabis Users. JAMA Psychiatry 2019, 76, 1305–1313. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.M.; Kaplan, B.L.F. Immune Responses Regulated by Cannabidiol. Cannabis Cannabinoid Res. 2020, 5, 12–31. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Cervantes, R.; Mendez-Diaz, M.; Prospero-Garcia, O.; Morales-Montor, J. Immunoregulatory Role of Cannabinoids during Infectious Disease. Neuroimmunomodulation 2017, 24, 183–199. [Google Scholar] [CrossRef]
- Hollister, L.E. Marijuana and immunity. J. Psychoact. Drugs 1992, 24, 159–164. [Google Scholar] [CrossRef]
- Marcellin, F.; Lions, C.; Rosenthal, E.; Roux, P.; Sogni, P.; Wittkop, L.; Protopopescu, C.; Spire, B.; Salmon-Ceron, D.; Dabis, F.; et al. No significant effect of cannabis use on the count and percentage of circulating CD4 T-cells in HIV-HCV co-infected patients (ANRS CO13-HEPAVIH French cohort). Drug Alcohol Rev. 2017, 36, 227–238. [Google Scholar] [CrossRef]
- Pacifici, R.; Zuccaro, P.; Farre, M.; Poudevida, S.; Abanades, S.; Pichini, S.; Langohr, K.; Segura, J.; de la Torre, R. Combined immunomodulating properties of 3,4-methylenedioxymethamphetamine (MDMA) and cannabis in humans. Addiction 2007, 102, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Bloom, J.W.; Kaltenborn, W.T.; Paoletti, P.; Camilli, A.; Lebowitz, M.D. Respiratory effects of non-tobacco cigarettes. Br. Med. J. 1987, 295, 1516–1518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tashkin, D.P.; Fligiel, S.; Wu, T.C.; Gong, H., Jr.; Barbers, R.G.; Coulson, A.H.; Simmons, M.S.; Beals, T.F. Effects of habitual use of marijuana and/or cocaine on the lung. In Research Findings on Smoking of Abused Substances; NIDA Research Monograph Series; Chiang, C.N., Hawks, R.L., Eds.; National Institute on Drug Abuse: Rochville, MD, USA, 1990; Volume 99, pp. 63–87. [Google Scholar]
- Yayan, J.; Rasche, K. Damaging Effects of Cannabis Use on the Lungs. Adv. Exp. Med. Biol. 2016, 952, 31–34. [Google Scholar] [PubMed]
- Tashkin, D.P.; Baldwin, G.C.; Sarafian, T.; Dubinett, S.; Roth, M.D. Respiratory and immunologic consequences of marijuana smoking. J. Clin. Pharmacol. 2002, 42, 71S–81S. [Google Scholar] [CrossRef]
- Sherrill, D.L.; Krzyzanowski, M.; Bloom, J.W.; Lebowitz, M.D. Respiratory effects of non-tobacco cigarettes: A longitudinal study in general population. Int. J. Epidemiol. 1991, 20, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Starr, K.; Renneker, M. A cytologic evaluation of sputum in marijuana smokers. J. Fam. Pract. 1994, 39, 359–363. [Google Scholar] [PubMed]
- Caiaffa, W.T.; Vlahov, D.; Graham, N.M.; Astemborski, J.; Solomon, L.; Nelson, K.E.; Munoz, A. Drug smoking, Pneumocystis carinii pneumonia, and immunosuppression increase risk of bacterial pneumonia in human immunodeficiency virus-seropositive injection drug users. Am. J. Respir. Crit. Care Med. 1994, 150, 1493–1498. [Google Scholar] [CrossRef]
- Bailey, K.L.; Wyatt, T.A.; Katafiasz, D.M.; Taylor, K.W.; Heires, A.J.; Sisson, J.H.; Romberger, D.J.; Burnham, E.L. Alcohol and cannabis use alter pulmonary innate immunity. Alcohol 2019, 80, 131–138. [Google Scholar] [CrossRef]
- Bredt, B.M.; Higuera-Alhino, D.; Shade, S.B.; Hebert, S.J.; McCune, J.M.; Abrams, D.I. Short-term effects of cannabinoids on immune phenotype and function in HIV-1-infected patients. J. Clin. Pharmacol. 2002, 42, 82S–89S. [Google Scholar] [CrossRef]
- Dong, C.; Chen, J.; Harrington, A.; Vinod, K.Y.; Hegde, M.L.; Hegde, V.L. Cannabinoid exposure during pregnancy and its impact on immune function. Cell. Mol. Life Sci. 2019, 76, 729–743. [Google Scholar] [CrossRef] [PubMed]
- Acharya, N.; Penukonda, S.; Shcheglova, T.; Hagymasi, A.T.; Basu, S.; Srivastava, P.K. Endocannabinoid system acts as a regulator of immune homeostasis in the gut. Proc. Natl. Acad. Sci. USA 2017, 114, 5005–5010. [Google Scholar] [CrossRef] [Green Version]
- Lowin, T.; Schneider, M.; Pongratz, G. Joints for joints: Cannabinoids in the treatment of rheumatoid arthritis. Curr. Opin. Rheumatol. 2019, 31, 271–278. [Google Scholar] [CrossRef]
- Katz-Talmor, D.; Katz, I.; Porat-Katz, B.S.; Shoenfeld, Y. Cannabinoids for the treatment of rheumatic diseases—Where do we stand? Nat. Rev. Rheumatol. 2018, 14, 488–498. [Google Scholar] [CrossRef]
- Naftali, T.; Lev, L.B.; Yablecovitch, D.; Half, E.; Konikoff, F.M. Treatment of Crohn’s disease with cannabis: An observational study. Isr. Med. Assoc. J. 2011, 13, 455–458. [Google Scholar] [PubMed]
- Naftali, T.; Mechulam, R.; Lev, L.B.; Konikoff, F.M. Cannabis for inflammatory bowel disease. Dig. Dis. 2014, 32, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Naftali, T.; Mechulam, R.; Marii, A.; Gabay, G.; Stein, A.; Bronshtain, M.; Laish, I.; Benjaminov, F.; Konikoff, F.M. Low-Dose Cannabidiol Is Safe but Not Effective in the Treatment for Crohn’s Disease, a Randomized Controlled Trial. Dig. Dis. Sci. 2017, 62, 1615–1620. [Google Scholar] [CrossRef]
- Picardo, S.; Kaplan, G.G.; Sharkey, K.A.; Seow, C.H. Insights into the role of cannabis in the management of inflammatory bowel disease. Ther. Adv. Gastroenterol. 2019, 12, 1756284819870977. [Google Scholar] [CrossRef]
- Riggs, P.K.; Vaida, F.; Rossi, S.S.; Sorkin, L.S.; Gouaux, B.; Grant, I.; Ellis, R.J. A pilot study of the effects of cannabis on appetite hormones in HIV-infected adult men. Brain Res. 2012, 1431, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Farokhnia, M.; McDiarmid, G.R.; Newmeyer, M.N.; Munjal, V.; Abulseoud, O.A.; Huestis, M.A.; Leggio, L. Effects of oral, smoked, and vaporized cannabis on endocrine pathways related to appetite and metabolism: A randomized, double-blind, placebo-controlled, human laboratory study. Transl. Psychiatry 2020, 10, 71. [Google Scholar] [CrossRef] [Green Version]
- Rom, S.; Persidsky, Y. Cannabinoid receptor 2: Potential role in immunomodulation and neuroinflammation. J. Neuroimmune Pharmacol. 2013, 8, 608–620. [Google Scholar] [CrossRef] [Green Version]
- Henriquez, J.E.; Rizzo, M.D.; Crawford, R.B.; Gulick, P.; Kaminski, N.E. Interferon-α-Mediated Activation of T Cells from Healthy and HIV-Infected Individuals Is Suppressed by Δ9-Tetrahydrocannabinol. J. Pharmacol. Exp. Ther. 2018, 367, 49–58. [Google Scholar] [CrossRef] [Green Version]
- Rizzo, M.D.; Henriquez, J.E.; Blevins, L.K.; Bach, A.; Crawford, R.B.; Kaminski, N.E. Targeting Cannabinoid Receptor 2 on Peripheral Leukocytes to Attenuate Inflammatory Mechanisms Implicated in HIV-Associated Neurocognitive Disorder. J. Neuroimmune Pharmacol. 2020, 15, 780–793. [Google Scholar] [CrossRef]
- Abrams, D.I.; Guzman, M. Cannabis in cancer care. Clin. Pharmacol. Ther. 2015, 97, 575–586. [Google Scholar] [CrossRef]
- Friedman, H.; Newton, C.; Klein, T.W. Microbial infections, immunomodulation, and drugs of abuse. Clin. Microbiol. Rev. 2003, 16, 209–219. [Google Scholar] [CrossRef] [Green Version]
- Manuzak, J.A.; Gott, T.M.; Kirkwood, J.S.; Coronado, E.; Hensley-McBain, T.; Miller, C.; Cheu, R.K.; Collier, A.C.; Funderburg, N.T.; Martin, J.N.; et al. Heavy Cannabis Use Associated with Reduction in Activated and Inflammatory Immune Cell Frequencies in Antiretroviral Therapy-Treated Human Immunodeficiency Virus-Infected Individuals. Clin. Infect. Dis. 2018, 66, 1872–1882. [Google Scholar] [CrossRef] [Green Version]
- Coates, R.A.; Farewell, V.T.; Raboud, J.; Read, S.E.; MacFadden, D.K.; Calzavara, L.M.; Johnson, J.K.; Shepherd, F.A.; Fanning, M.M. Cofactors of progression to acquired immunodeficiency syndrome in a cohort of male sexual contacts of men with human immunodeficiency virus disease. Am. J. Epidemiol. 1990, 132, 717–722. [Google Scholar] [CrossRef]
- Kaslow, R.A.; Blackwelder, W.C.; Ostrow, D.G.; Yerg, D.; Palenicek, J.; Coulson, A.H.; Valdiserri, R.O. No evidence for a role of alcohol or other psychoactive drugs in accelerating immunodeficiency in HIV-1-positive individuals. A report from the Multicenter AIDS Cohort Study. JAMA 1989, 261, 3424–3429. [Google Scholar] [CrossRef]
- Watson, C.W.; Paolillo, E.W.; Morgan, E.E.; Umlauf, A.; Sundermann, E.E.; Ellis, R.J.; Letendre, S.; Marcotte, T.D.; Heaton, R.K.; Grant, I. Cannabis Exposure is Associated with a Lower Likelihood of Neurocognitive Impairment in People Living with HIV. J. Acquir. Immune Defic. Syndr. 2020, 83, 56–64. [Google Scholar] [CrossRef]
- Lutge, E.E.; Gray, A.; Siegfried, N. The medical use of cannabis for reducing morbidity and mortality in patients with HIV/AIDS. Cochrane Database Syst. Rev. 2013. [Google Scholar] [CrossRef]
- Lake, S.; Kerr, T.; Capler, R.; Shoveller, J.; Montaner, J.; Milloy, M.J. High-intensity cannabis use and HIV clinical outcomes among HIV-positive people who use illicit drugs in Vancouver, Canada. Int. J. Drug Policy 2017, 42, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Wardell, J.D.; Shuper, P.A.; Hendershot, C.S. A longitudinal investigation of the association between cannabis use and alcohol use among people living with HIV. Drug Alcohol Depend. 2018, 193, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Bonn-Miller, M.O.; Oser, M.L.; Bucossi, M.M.; Trafton, J.A. Cannabis use and HIV antiretroviral therapy adherence and HIV-related symptoms. J. Behav. Med. 2014, 37, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Mannes, Z.L.; Burrell, L.E., II; Ferguson, E.G.; Zhou, Z.; Lu, H.; Somboonwit, C.; Cook, R.L.; Ennis, N. The association of therapeutic versus recreational marijuana use and antiretroviral adherence among adults living with HIV in Florida. Patient Prefer. Adherence 2018, 12, 1363–1372. [Google Scholar] [CrossRef] [Green Version]
- Slawson, G.; Milloy, M.J.; Balneaves, L.; Simo, A.; Guillemi, S.; Hogg, R.; Montaner, J.; Wood, E.; Kerr, T. High-intensity cannabis use and adherence to antiretroviral therapy among people who use illicit drugs in a Canadian setting. AIDS Behav. 2015, 19, 120–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woolridge, E.; Barton, S.; Samuel, J.; Osorio, J.; Dougherty, A.; Holdcroft, A. Cannabis use in HIV for pain and other medical symptoms. J. Pain Symptom Manag. 2005, 29, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Wilson, N.L.; Peterson, S.N.; Ellis, R.J. Cannabis and the Gut-Brain Axis Communication in HIV Infection. Cannabis Cannabinoid Res. 2021, 6, 92–104. [Google Scholar] [CrossRef]
- Suryavanshi, S.V.; Kovalchuk, I.; Kovalchuk, O. Cannabinoids as Key Regulators of Inflammasome Signaling: A Current Perspective. Front. Immunol. 2020, 11, 613613. [Google Scholar] [CrossRef]
- Hezode, C.; Roudot-Thoraval, F.; Nguyen, S.; Grenard, P.; Julien, B.; Zafrani, E.S.; Pawlotsky, J.M.; Dhumeaux, D.; Lotersztajn, S.; Mallat, A. Daily cannabis smoking as a risk factor for progression of fibrosis in chronic hepatitis C. Hepatology 2005, 42, 63–71. [Google Scholar] [CrossRef]
- Ishida, J.H.; Peters, M.G.; Jin, C.; Louie, K.; Tan, V.; Bacchetti, P.; Terrault, N.A. Influence of cannabis use on severity of hepatitis C disease. Clin. Gastroenterol. Hepatol. 2008, 6, 69–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wijarnpreecha, K.; Panjawatanan, P.; Ungprasert, P. Use of cannabis and risk of advanced liver fibrosis in patients with chronic hepatitis C virus infection: A systematic review and meta-analysis. J. Evid. Based Med. 2018, 11, 272–277. [Google Scholar] [CrossRef]
- Kelly, E.M.; Dodge, J.L.; Sarkar, M.; French, A.L.; Tien, P.C.; Glesby, M.J.; Golub, E.T.; Augenbraun, M.; Plankey, M.; Peters, M.G. Marijuana Use Is Not Associated With Progression to Advanced Liver Fibrosis in HIV/Hepatitis C Virus-coinfected Women. Clin. Infect. Dis. 2016, 63, 512–518. [Google Scholar] [CrossRef] [Green Version]
- Nordmann, S.; Vilotitch, A.; Roux, P.; Esterle, L.; Spire, B.; Marcellin, F.; Salmon-Ceron, D.; Dabis, F.; Chas, J.; Rey, D.; et al. Daily cannabis and reduced risk of steatosis in human immunodeficiency virus and hepatitis C virus-co-infected patients (ANRS CO13-HEPAVIH). J. Viral Hepat. 2018, 25, 171–179. [Google Scholar] [CrossRef]
- Archie, S.R.; Cucullo, L. Cerebrovascular and Neurological Dysfunction under the Threat of COVID-19: Is There a Comorbid Role for Smoking and Vaping? Int. J. Mol. Sci. 2020, 21, 3916. [Google Scholar] [CrossRef]
- Nakagawa, M.; Nakahara, K.; Maruyama, Y.; Kawabata, M.; Higuchi, I.; Kubota, H.; Izumo, S.; Arimura, K.; Osame, M. Therapeutic trials in 200 patients with HTLV-I-associated myelopathy/ tropical spastic paraparesis. J. Neurovirol. 1996, 2, 345–355. [Google Scholar] [CrossRef]
- Araujo, A.; Lima, M.A.; Silva, M.T. Human T-lymphotropic virus 1 neurologic disease. Curr. Treat. Options Neurol. 2008, 10, 193–200. [Google Scholar] [CrossRef]
- Rajabalendaran, N.; Burns, R.; Mollison, L.C.; Blessing, W.; Kirubakaran, M.G.; Lindschau, P. Tropical spastic paraparesis in an aborigine. Med. J. Aust. 1993, 159, 28–29. [Google Scholar] [CrossRef]
- Oliveira-Filho, A.B.; Araujo, A.P.S.; Souza, A.P.C.; Gomes, C.M.; Silva-Oliveira, G.C.; Martins, L.C.; Fischer, B.; Machado, L.F.A.; Vallinoto, A.C.R.; Ishak, R.; et al. Human T-lymphotropic virus 1 and 2 among people who used illicit drugs in the state of Para, northern Brazil. Sci. Rep. 2019, 9, 14750. [Google Scholar] [CrossRef] [Green Version]
- Sarafian, T.A.; Kouyoumjian, S.; Khoshaghideh, F.; Tashkin, D.P.; Roth, M.D. Δ9-tetrahydrocannabinol disrupts mitochondrial function and cell energetics. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003, 284, L298–L306. [Google Scholar] [CrossRef] [Green Version]
- Beji, C.; Loucif, H.; Telittchenko, R.; Olagnier, D.; Dagenais-Lussier, X.; van Grevenynghe, J. Cannabinoid-Induced Immunomodulation during Viral Infections: A Focus on Mitochondria. Viruses 2020, 12, 875. [Google Scholar] [CrossRef] [PubMed]
- Gorbach, P.M.; Javanbakht, M.; Shover, C.L.; Bolan, R.K.; Ragsdale, A.; Shoptaw, S. Associations Between Cannabis Use, Sexual Behavior, and Sexually Transmitted Infections/Human Immunodeficiency Virus in a Cohort of Young Men Who Have Sex with Men. Sex. Transm. Dis. 2019, 46, 105–111. [Google Scholar] [CrossRef]
- Khalsa, J.H.; Bunt, G.; Maggirwar, S.B.; Kottilil, S. COVID-19 and Cannabidiol (CBD). J. Addict. Med. 2020. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maggirwar, S.B.; Khalsa, J.H. The Link between Cannabis Use, Immune System, and Viral Infections. Viruses 2021, 13, 1099. https://doi.org/10.3390/v13061099
Maggirwar SB, Khalsa JH. The Link between Cannabis Use, Immune System, and Viral Infections. Viruses. 2021; 13(6):1099. https://doi.org/10.3390/v13061099
Chicago/Turabian StyleMaggirwar, Sanjay B., and Jag H. Khalsa. 2021. "The Link between Cannabis Use, Immune System, and Viral Infections" Viruses 13, no. 6: 1099. https://doi.org/10.3390/v13061099