Imaging and Assessment of the Microstructure of Conserved Archaeological Pine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
- 1.
- Reference
- 2.
- Alkohol-ether resin
- 3.
- Melamine formaldehyde (Kauramin 800®, BASF, Ludwigshafen, Germany)
- 4.
- Lactitol-trehalose
- 5.
- Polyethylene glycol (PEG 2000) one-step and freeze-drying
- 6.
- Polyethylene glycol (PEG 400 and 4000) two-step and freeze-drying
- 7.
- Polyethylene glycol (PEG 400, 1500 and 4000) three-step and freeze-drying
- 8.
- Saccharose
- 9.
- Silicone oil
2.2. Methods
2.2.1. Optical Microscope
2.2.2. Computed Tomography
2.2.3. Scanning Electron Microscope
3. Results
3.1. Air-Dried Reference Sample
3.2. Alcohol-Ether-Resin
3.3. Melamine Formaldehyde (Kauramin 800)
3.4. Lactitol/Trehalose
3.5. PEG 2000 and Freeze-Drying
3.6. PEG 400, PEG 4000 and Freeze-Drying
3.7. PEG 400, PEG 1500, PEG 4000 and Freeze-Drying
3.8. Saccharose
3.9. Silicone Oil
4. Discussion
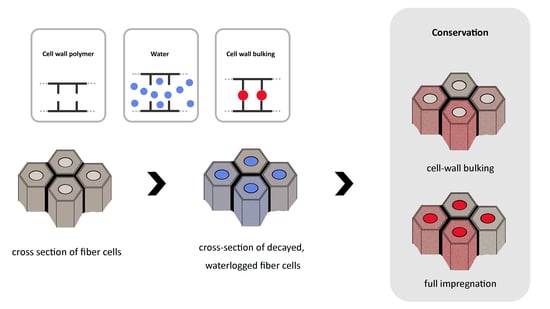
4.1. Distribution of the Conservation Agent
4.2. Relationship between Microscopic and Macroscopic Level
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Conservation Method | Institutions and Short Descriptions of the Methods | |
|---|---|---|
| Air dried reference | Institution: | Leibniz-Zentrum für Archäologie, Mainz, Germany |
| Treatment: | none, air-drying | |
| Impregnation solution: | none | |
| Alcohol-ether-resin | Institution: | Schweizerisches Nationalmuseum, Zürich, Switzerland. |
| Treatment: | Exchange of water with ethanol. Exchange of ethanol with diethyl ether. Soaking of wood with diethyl ether in resin-diethyl solution. Drying by evaporation of the diethyl ether in the vacuum vessel. Application of surface protection 3% Paraloid B72 solution in acetone. | |
| Impregnation solution: | 70.7% diethyl ether, 16.1% dammar resin, 6.4% rosin, 3.2% dienol D102, 3.2% rhizinus oil, 0.4% PEG 400 | |
| Kauramin 800 | Institution: | Leibniz-Zentrum für Archäologie, Mainz, Germany |
| Treatment: | Soaking in demineralized water. Bath impregnation at room temperature. Replacement of the solution when early polymerisation occurs. Curing of the impregnated wood in the heating cabinet at 60 °C. Afterwards slow, controlled air-drying. Dip in linseed oil varnish. | |
| Impregnation solution: | 25% Kauramin 800 solution (72 L resin + 210 L deionised water, 3.6 L urea, 7.2 L triethylene glycol) | |
| Lactitol-trehalose | Institution: | Brandenburgisches Landesamt für Denkmalpflege, Zossen, Germany |
| Treatment: | Starting with 30% concentration. Increasing monthly in 10% steps up to 70%. Bath temperature 55 °C. After removal from the bath, the surfaces were dusted with crystalline lactitol monohydrate and dried in a heating oven over a period of one week. After drying, the surface was cleaned by dabbing with damp cloths. | |
| Impregnation solution: | lactitol-trehalose solution (9:1) 30%–70%. Addition of biocide if necessary (0,1% Bioban 404) | |
| Polyethylene glycol (PEG 2000) one-step and freeze-drying | Institution: | Nationalmuseet, Copenhagen, Denmark |
| Treatment: | Starting with 10% PEG 2000 solution. Increasing the concentration up to 40% at room temperature. Freeze-drying in cooled chamber (approx. −30 °C). Removal of excess PEG from the surface with a soft brush and ethanol. Subsequent surface stabilisation with 25% PEG 2000 solution in ethanol. | |
| Impregnation solution: | PEG 2000, 10–40% solution with tap water | |
| Polyethylene glycol (PEG 400 and 4000) two-step and freeze-drying | Institution: | Brandenburgisches Landesamt für Denkmalpflege, Zossen, Germany |
| Treatment: | Soaking in demineralised water. Starting with 5% PEG 400 solution. Raising the concentration in 5% steps every 4 weeks. At its calculated final concentration, it was kept constant. Then the increase of PEG 4000 solution was continued in 5% steps up to its final concentration where it was kept for 10 weeks. Precooling of the wood to 5 °C then deep-freezing to −25 to −35 °C, freeze-drying in cooled chamber (approx. −30 °C). | |
| Impregnation solution: | PEG-solution in demineralised water (PEG 400 and PEG 4000) was adapted according to the condition of the wood (PEGcon): 35% PEG solution (of which 10% PEG 400 and 25% PEG 4000) in demineralized water, impregnation at room temperature | |
| Polyethylene glycol (PEG 400, 1500 and 4000) three-step and freeze-drying | Institution: | Archäologische Staatssammlung, Munich, Germany |
| Treatment: | Soaking in demineralised water. Starting with 11% increasing to 15% PEG 400 solution at room temperature. 16% increasing to 20.5% PEG 1500 solution at 40 °C. 20.5% increasing to 27.5% PEG 4000 solution at 40 °C. Washing of the wood and wrapping in cellulose tissues. Intermediate storage in freezer (−25 to −35 °C) until freeze-drying. Subsequent freeze-drying in a cooled chamber (approx. −30 °C). Excess of PEG was removed with a brush and ethanol. | |
| Impregnation solution: | PEG-solution in demineralised water: 15% PEG 400, 20.5% PEG 1500, 27.5% PEG 4000 | |
| Saccharose | Institution: | Sächsisches Landesamt für Archäologie, Dresden, Germany |
| Treatment: | Concentrated the solution in 10% steps, from 10% up to 60% sugar solution at room temperature. Slow, controlled air-drying in microperforated bags. Removal of crystallised sugar residues from the surface with damp sponge. | |
| Impregnation solution: | Aqueous saccharose solution 10%–60%. If necessary biocide addition composed of 0.6%, sodium benzoate (E211), 0.5% Parmetol K40, 0.5% Quartasept Plus and 0.02% Tallofin OT | |
| Silicone oil | Institution: | Texas University, Texas, USA |
| Treatment: | Exchange of water with ethanol. Exchange of ethanol with acetone. Placing the still dripping wet acetone-impregnated samples in impregnation solution under normal atmospheric conditions. Triggering the polymerisation of the impregnation solution by gaseous catalyst: DBTDA (dibutyl diacetate). | |
| Impregnation solution: | 80% silicone oil (SFD1 (66%) + SFD5 (34%)—silanol functional polydimethylsiloxanes “PDMS”) and 20% crosslinker MTMS (methyltrimethoxysilane) | |
| Sample | Database [33] | Conservation | Condition | Analysis | ||
|---|---|---|---|---|---|---|
| CT | SEM | LM | ||||
| Pi-Air-ld | V03-01 | None, air-drying | Low degraded | x | x | |
| Pi-Air-hd | heavily degraded | x | x | x | ||
| Pi-AlEt-ld | V03-17 | Alcohol-ether-resin | Low degraded | x | ||
| Pi-AlEt-hd | heavily degraded | x | x | x | ||
| Pi-K800-ld | V03-41 | Melamine formaldehyde | Low degraded | x | x | |
| Pi-K800-hd | heavily degraded | x | x | x | ||
| Pi-LaTr-ld | V03-28 | Lactitol-trehalose | Low degraded | x | x | |
| Pi-LaTr-hd | heavily degraded | x | x | x | ||
| Pi-PEG1-ld | V03-35 | PEG 2000 | Low degraded | x | x | |
| Pi-PEG1-hd | heavily degraded | x | x | x | ||
| Pi-PEG2-ld | V03-32 | PEG 400 and 4000 | Low degraded | x | x | |
| Pi-PEG2-hd | heavily degraded | x | x | x | ||
| Pi-PEG3-ld | V03-20 | PEG 400, 1500 and 4000 | Low degraded | x | x | |
| Pi-PEG3-hd | heavily degraded | x | x | x | ||
| Pi-Sac-ld | V03-45 | Saccharose | Low degraded | x | x | |
| Pi-Sac-hd | heavily degraded | x | x | x | ||
| Pi-Sil-ld | V03-42 | Silicone oil | Low degraded | x | x | |
| Pi-Sil-hd | heavily degraded | x | x | x | ||
References
- Broda, M.; Hill, C.A.S. Conservation of Waterlogged Wood—Past, Present and Future Perspectives. Forests 2021, 12, 1193. [Google Scholar] [CrossRef]
- Gregory, D.; Jensen, P. The Importance of Analysing Waterlogged Wooden Artefacts and Environmental Conditions When Considering Their In Situ Preservation. J. Wetl. Archaeol. 2006, 6, 65–81. [Google Scholar] [CrossRef]
- Lucejko, J.J.; Tamburini, D.; Modugno, F.; Ribechini, E.; Colombini, M.P. Analytical Pyrolysis and Mass Spectrometry to Characterise Lignin in Archaeological Wood. Appl. Sci. 2021, 11, 240. [Google Scholar] [CrossRef]
- Pournou, A. Biodeterioration of Wooden Cultural Heritage: Organisms and Decay Mechanisms in Aquatic and Terrestrial Ecosystems; Springer: Cham, Switzerland, 2020; ISBN 978-3-030-46503-2. [Google Scholar]
- Walsh-Korb, Z.; Avérous, L. Recent Developments in the Conservation of Materials Properties of Historical Wood. Prog. Mater. Sci. 2019, 102, 167–221. [Google Scholar] [CrossRef]
- Zisi, A. Forest Wood through the Eyes of a Cultural Conservator. Forests 2021, 12, 1001. [Google Scholar] [CrossRef]
- Björdal, C.G.; Nilsson, T.; Daniel, G. Microbial Decay of Waterlogged Archaeological Wood Found in Sweden Applicable to Archaeology and Conservation. Int. Biodeterior. Biodegrad. 1999, 43, 63–73. [Google Scholar] [CrossRef]
- Klaassen, R.K.W.M. Bacterial Decay in Wooden Foundation Piles—Patterns and Causes: A Study of Historical Pile Foundations in the Netherlands. Int. Biodeterior. Biodegrad. 2008, 61, 45–60. [Google Scholar] [CrossRef]
- Pedersen, N.B.; Łucejko, J.J.; Modugno, F.; Björdal, C. Correlation between Bacterial Decay and Chemical Changes in Waterlogged Archaeological Wood Analysed by Light Microscopy and Py-GC/MS. Holzforschung 2021, 75, 635–645. [Google Scholar] [CrossRef]
- Singh, A.P.; Kim, Y.S.; Chavan, R.R. Advances in Understanding Microbial Deterioration of Buried and Waterlogged Archaeological Woods: A Review. Forests 2022, 13, 394. [Google Scholar] [CrossRef]
- Grosser, D. Die Hölzer Mitteleuropas: Ein Mikrophotographischer Lehratlas; Springer: Berlin, Germany, 1977; ISBN 978-3-540-08096-1. [Google Scholar]
- Fengel, D.; Wegener, G. Wood: Chemistry, Ultrastructure, Reactions; Walter de Gruyter: Berlin, Germany, 2011; ISBN 978-3-11-083965-4. [Google Scholar]
- Zhang, X.; Li, L.; Xu, F. Chemical Characteristics of Wood Cell Wall with an Emphasis on Ultrastructure: A Mini-Review. Forests 2022, 13, 439. [Google Scholar] [CrossRef]
- Hawley, L.F. Wood-Liquid Relations. Tech. Bull. 1931, 248, 1–34. [Google Scholar]
- Broda, M.; Curling, S.F.; Frankowski, M. The Effect of the Drying Method on the Cell Wall Structure and Sorption Properties of Waterlogged Archaeological Wood. Wood Sci. Technol. 2021, 55, 971–989. [Google Scholar] [CrossRef]
- Stelzner, J.; Stelzner, I.; Martinez-Garcia, J.; Gwerder, D.; Wittkoepper, M.; Muskalla, W.; Cramer, A.; Heinz, G.; Egg, M.; Schuetz, P. Stabilisation of Waterlogged Archaeological Wood—The Application of Structured-Light 3D Scanning and Micro Computed Tomography for Analysing Dimensional Changes. Herit. Sci. 2022, 10, 1–25. [Google Scholar] [CrossRef]
- Grattan, D.W.; Clarke, R.W. Conservation of Waterlogged Wood. In Conservation of Marine Archaeological Objects; Pearson, C., Ed.; Butterworth-Heinemann: Oxford, UK, 1987; pp. 164–206. ISBN 978-0-408-10668-9. [Google Scholar]
- Schnell, U.; Jensen, P. Determination of Maximum Freeze Drying Temperature for PEG-Impregnated Archaeological Wood. Stud. Conserv. 2007, 52, 50–58. [Google Scholar] [CrossRef]
- Parrent, J.M. The Conservation of Waterlogged Wood Using Sucrose. Stud. Conserv. 1985, 30, 63–72. [Google Scholar] [CrossRef]
- Jensen, P.; Gregory, D.J. Selected Physical Parameters to Characterize the State of Preservation of Waterlogged Archaeological Wood: A Practical Guide for Their Determination. J. Archaeol. Sci. 2006, 33, 551–559. [Google Scholar] [CrossRef]
- Walsh-Korb, Z.; Stelzner, I.; Dos Santos Gabriel, J.; Eggert, G.; Avérous, L. Morphological Study of Bio-Based Polymers in the Consolidation of Waterlogged Wooden Objects. Mater. Basel Switz. 2022, 15, 681. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M. Developing New Consolidants for Archaeological Wood. Ph.D. Thesis, University of Oslo, Oslo, Norway, 2013. [Google Scholar]
- Bräker, O.U.; Bill, J.; Mühlethaler, B.; Schoch, W.; Schweingruber, F.H.; Haas, A.; Hug, B.; Kramer, W.; Elmer, J.T.; Tassigny, C.D.; et al. Zum derzeitigen Stand der Nassholzkonservierung. Diskussion der Grundlagen und Resultate eines von Fachlaboratorien 1976-1978 durchgeführten Methodenvergleiches. Z. Für Schweiz. Archaeol. Kunstgesch. 1979, 36, 97–145. [Google Scholar]
- Jensen, P.; Petersen, A.H.; Straetkvern, K. From the Skuldelev to the Roskilde Ships—50 Years of Shipwreck Conservation at the National Musem of Denmark. In Proceedings of the Shipwrecks 2011 Chemistry and Preservation of Waterlogged Wooden Shipwrecks; Ek, M., Ed.; ICOM-CC: Stockholm, Sweden, 2011; pp. 14–20. [Google Scholar]
- Müller-Beck, H.-J.; Haas, A. A Method for Wood Preservation Using Arigal C. Stud. Conserv. 1960, 5, 150–158. [Google Scholar]
- Wittköpper, M. Der aktuelle Stand der Konservierung archäologischer Naßhölzer mit Melamin/Aminoharzen am Römisch-Germanischen Zentralmuseum. Arbeitsblätter Restaur. 1998, 31, 227–283. [Google Scholar]
- Babiński, L. Dimensional Changes of Waterlogged Archaeological Hardwoods Pre-Treated with Aqueous Mixtures of Lactitol/Trehalose and Mannitol/Trehalose before Freeze-Drying. J. Cult. Herit. 2015, 16, 876–882. [Google Scholar] [CrossRef]
- Han, L.; Guo, J.; Tian, X.; Jiang, X.; Yin, Y. Evaluation of PEG and Sugars Consolidated Fragile Waterlogged Archaeological Wood Using Nanoindentation and ATR-FTIR Imaging. Int. Biodeterior. Biodegrad. 2022, 170, 105390. [Google Scholar] [CrossRef]
- Hoffmann, P. Zur Naßholzkonservierung mit Zucker am Deutschen Schiffahrtsmuseum eine Bilanz. Arbeitsblätter der Restauratoren 1996, 10, 231–240. [Google Scholar]
- Imazu, S.; Morgós, A. An Improvement on the Lactitol MC Conservation Method Used for the Conservation of Archaeological Waterlogged Wood (The Conservation Method Using Lactitol MC and Trehalose Mixture). In Proceedings of the 8th ICOM Group on Wet Organic Archaeological Materials Conference, Stockholm, Sweden, 11–15 June 2001; Hoffmann, P., Spriggs, J.A., Grant, T., Cook, C., Recht, A., Eds.; ICOM Committee for Conservation: Bremerhaven, Germany, 2002; pp. 413–428. [Google Scholar]
- Jensen, P.; Pedersen, N.B. Examination of D-Mannitol as an Impregnation Agent for Heavily Degraded Waterlogged Archaeological Wood. In Proceedings of the 12th ICOM-CC Group on Wet Organic Archaeological Materials Conference, Istanbul, Turkey, 13–17 May 2013; Grant, T., Cook, C., Eds.; ICOM-CC: Istanbul, Turkey, 2016; pp. 118–125. [Google Scholar]
- Kennedy, A.; Pennington, E.R. Conservation of Chemically Degraded Waterlogged Wood with Sugars. Stud. Conserv. 2014, 59, 194–201. [Google Scholar] [CrossRef]
- Imazu, S.; Ito, K.; Fujita, H.; Morgos, A. The Rapid Trehalose Conservation Method for Archaeological Waterlogged Wood and Laquerware. In Proceedings of the 12th ICOM-CC Group on Wet Organic Archaeological Materials Conference, Istanbul, Turkey, 13–17 May 2013; Grant, T., Cook, C., Eds.; ICOM-CC: Istanbul, Turkey, 2016; pp. 110–117. [Google Scholar]
- Broda, M.; Dąbek, I.; Dutkiewicz, A.; Dutkiewicz, M.; Popescu, C.-M.; Mazela, B.; Maciejewski, H. Organosilicons of Different Molecular Size and Chemical Structure as Consolidants for Waterlogged Archaeological Wood—A New Reversible and Retreatable Method. Sci. Rep. 2020, 10, 2188. [Google Scholar] [CrossRef]
- Hamilton, D.L. Methods of Conserving Archaeological Material from Underwater Sites, 2nd ed.; Anthropology; Department of Anthropology, Texas A&M University, Ed.; Conservation of Archaeological Resources I, Texas A&M University: Texas TX, USA, 2010. [Google Scholar]
- Smith, C.W. Archaeological Conservation Using Polymers: Practical Applications for Organic Artifact Stabilization; Texas A&M University Press: Texas TX, USA, 2003; ISBN 978-1-58544-217-1. [Google Scholar]
- Hocker, E.; Almkvist, G.; Sahlstedt, M. The Vasa Experience with Polyethylene Glycol: A Conservator’s Perspective. J. Cult. Herit. 2012, 13, S175–S182. [Google Scholar] [CrossRef]
- Hoffmann, P. A Rapid Method for the Detection of Polyethylene Glycols (Peg) in Wood. Stud. Conserv. 1983, 28, 189–193. [Google Scholar] [CrossRef]
- Schmidt-Ott, K.; André, C.; Bader, M. Fishing for Stability: Conserving a Fish Trap in a Block Excavation by the Alcohol-Ether-Resin Method. In Proceedings of the 14th ICOM-CC Wet Organic Archaeological Materials Working Group Interim Meeting, Portsmouth, UK, 20–24 May 2019; ICOM-CC: Portsmouth, UK, 2022; pp. 322–327. [Google Scholar]
- Jensen, P.; Strætkvern, K.; Schnell, U.; Jensen, J.B. Technical Specifications for Equipment for Vacuum Freeze-Drying of PEG Impregnated Waterlogged Organic Materials. In Proceedings of the 10th ICOM-CC Group on Wet Organic Archaeological Materials Conference, Amsterdam, The Netherlands, 10–15 September 2007; ICOM-CC: Amsterdam, The Netherlands, 2010; pp. 417–438. [Google Scholar]
- Wiesner, I.; Gieseler, H. Freeze-Dry Microscopy—Real-Time Observation of the Drying Process. In Proceedings of the 12th ICOM-CC Wet Organic Archaeological Materials Conference, Istanbul, Turkey, 13–17 May 2013; Grant, T., Cook, C., Eds.; ICOM-CC: Istanbul, Turkey, 2016; pp. 417–424. [Google Scholar]
- Massenfunde in archäologischen Sammlungen. Available online: www.rgzm.de/kur (accessed on 1 December 2022).
- Altgen, M.; Awais, M.; Altgen, D.; Klüppel, A.; Mäkelä, M.; Rautkari, L. Distribution and Curing Reactions of Melamine Formaldehyde Resin in Cells of Impregnation-Modified Wood. Sci. Rep. 2020, 10, 3366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, P. Conservation of Archaeological Ships and Boats: Personal Experiences; Archetype Publications: London, UK, 2013; ISBN 978-1-904982-82-1. [Google Scholar]
- Daniel, G. Microscope Techniques for Understanding Wood Cell Structure and Biodegradation. In Secondary Xylem Biology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 309–343. ISBN 978-0-12-802185-9. [Google Scholar]
- Giachi, G.; Capretti, C.; Donato, I.D.; Macchioni, N.; Pizzo, B. New Trials in the Consolidation of Waterlogged Archaeological Wood with Different Acetone-Carried Products. J. Archaeol. Sci. 2011, 38, 2957–2967. [Google Scholar] [CrossRef]
- Stelzner, J.; Million, S. X-Ray Computed Tomography for the Anatomical and Dendrochronological Analysis of Archaeological Wood. J. Archaeol. Sci. 2015, 55, 188–196. [Google Scholar] [CrossRef]
- Bugani, S.; Modugno, F.; Łucejko, J.J.; Giachi, G.; Cagno, S.; Cloetens, P.; Janssens, K.; Morselli, L. Study on the Impregnation of Archaeological Waterlogged Wood with Consolidation Treatments Using Synchrotron Radiation Microtomography. Anal. Bioanal. Chem. 2009, 395, 1977–1985. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.; Hansen, F.K.; Kutzke, H. Phenol Formaldehyde Revisited-Novolac Resins for the Treatment of Degraded Archaeological Wood: Novolac Resins for Treatment of Degraded Archaeological Wood. Archaeometry 2015, 57, 536–559. [Google Scholar] [CrossRef]
- Braovac, S.; Sahlstedt, M.; Wittköpper, M. Retreatment Testing of Alum-Treated Woods from the Oseberg Collection—Results from First Trials. Wet Organic Archaeological Materials 2019. In Proceedings of the 14th ICOM-CC Wet Organic Archaeological Materials Working Group Interim Meeting, Portsmouth, UK, 20–24 May 2019; Williams, E., Ed.; ICOM-CC: Portsmouth, UK, 2022; pp. 207–215. [Google Scholar]
- Wittköpper, M.; Muskalla, W.; Stephan, B.; Le Boedec-Moesgard, A.; Gebhadt, S.; Klonk, S.; André, C.; Schmidt-Ott, K.; Smith, W. The KUR (Conservation and Restauration) Project—A Comparison of Different Methods to Preserve Waterlogged Wood. In Proceedings of the 12th ICOM-CC Group on Wet Organic Archaeological Materials Conference, Istanbul, Turkey, 13–17 May 2013; Grant, T., Cook, C., Eds.; ICOM-CC: Istanbul, Turkey, 2016; pp. 134–143. [Google Scholar]
- de Jong, J. The Conservation of Waterlogged Timber at Ketelhaven (Holland). In Proceedings of the 4th ICOM-CC Triennial Meeting, Venice, Italy, 13–18 October 1975; ICOM Committee for Conservation, Ed.; ICOM-CC: Paris, France, 1975; p. 75/8/1-1–9. [Google Scholar]
- High, K.E.; Penkman, K.E.H. A Review of Analytical Methods for Assessing Preservation in Waterlogged Archaeological Wood and Their Application in Practice. Herit. Sci. 2020, 8, 83. [Google Scholar] [CrossRef]
- Macchioni, N.; Pecoraro, E.; Pizzo, B. The Measurement of Maximum Water Content (MWC) on Waterlogged Archaeological Wood: A Comparison between Three Different Methodologies. J. Cult. Herit. 2018, 30, 51–56. [Google Scholar] [CrossRef]
- Scott, D.A.; Eggert, G. The Vicissitudes of Vivianite as Pigment and Corrosion Product. Stud. Conserv. 2007, 52, 3–13. [Google Scholar] [CrossRef]
- Macchioni, N.; Pizzo, B.; Capretti, C.; Giachi, G. How an Integrated Diagnostic Approach Can Help in a Correct Evaluation of the State of Preservation of Waterlogged Archaeological Wooden Artefacts. J. Archaeol. Sci. 2012, 39, 3255–3263. [Google Scholar] [CrossRef]
- Zborowska, M.; Babiński, L.; Gajewska, J.; Waliszewska, B.; Prądzyński, W. Pysical and Chemical Properties of Contemporary Pine Wood (Pinus Sylvestris L.) in Conditions of a Wet Archaeolocical Site in Biskupin. Folia For. Pol. 2007, 38, 13–26. [Google Scholar]
- McLean, P. Wood Properties and Uses of Scots Pine in Britain. Research Report; Forestry Commission: Edinburgh, UK, 2019. [Google Scholar]
- Feldkamp, L.A.; Davis, L.C.; Kress, J.W. Practical Cone-Beam Algorithm. JOSA A 1984, 1, 612–619. [Google Scholar] [CrossRef] [Green Version]
- Čufar, K.; Gričar, J.; Zupančič, M.; Koch, G.; Schmitt, U. Anatomy, Cell Wall Structure and Topochemistry of Water-Logged Archaeological Wood Aged 5,200 and 4,500 Years. IAWA J. 2008, 29, 55–68. [Google Scholar] [CrossRef] [Green Version]
- Bardet, M.; Gerbaud, G.; Trân, Q.-K.; Hediger, S. Study of Interactions between Polyethylene Glycol and Archaeological Wood Components by 13C High-Resolution Solid-State CP-MAS NMR. J. Archaeol. Sci. 2007, 34, 1670–1676. [Google Scholar] [CrossRef]
- Björdal, C.G.; Nilsson, T. Waterlogged Archaeological Wood—A Substrate for White Rot Fungi during Drainage of Wetlands. Int. Biodeterior. Biodegrad. 2002, 50, 17–23. [Google Scholar] [CrossRef]
- Björdal, C.G.; Nilsson, T. Observations on Microbial Growth during Conservation Treatment of Waterlogged Archaeological Wood. Stud. Conserv. 2001, 46, 211–220. [Google Scholar] [CrossRef]
- Guo, J.; Xiao, L.; Han, L.; Wu, H.; Yang, T.; Wu, S.; Yin, Y. Deterioration of the Cell Wall in Waterlogged Wooden Archeological Artifacts, 2400 Years Old. IAWA J. 2019, 40, 820–844. [Google Scholar] [CrossRef]
- Pedersen, N.B. Microscopic and Spectroscopic Characterisation of Waterlogged Archaeological Softwood from Anoxic Environments. Ph.D. Thesis, University of Copenhagen, Copenhagen, Denmark, 2015. [Google Scholar]
- Schweingruber, F.H. Mikroskopische Holzanatomie; 3. Auflage; Eidgenössische Forschungsanstalt für Wald, Schnee und Landschaft: Birmensdorf, Switzerland, 1990. [Google Scholar]
- Haas, A.; Müller-Beck, H.-J.; Schweingruber, F.H. Erfahrungen Bei Der Konservierung von Feuchthölzern Mit Arigal C. In Jahrbuch des Bernischen Historischen Museums in Bern; 1961/62; 41/42; 1961; pp. 509–537. [Google Scholar]
- Spinella, A.; Chillura Martino, D.F.; Saladino, M.L.; Sammartino, F.; Caruso, F.; Caponetti, E. Solid State NMR Investigation of the Roman Acqualadroni Rostrum: Tenth Year Assessment of the Consolidation Treatment of the Wooden Part. Cellulose 2021, 28, 1025–1038. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Kohdzuma, Y.; Endo, R.; Sugiyama, J. Evaluation of Chemical Treatments on Dimensional Stabilization of Archeological Waterlogged Hardwoods Obtained from the Thang Long Imperial Citadel Site, Vietnam. J. Wood Sci. 2018, 64, 436–443. [Google Scholar] [CrossRef] [Green Version]
- Bilz, M.; Grant, T.; Young, G. Treating Waterlogged Basketry: A Study of Polyethylene Glycol Penetration into the Inner Bark of Western Red Cedar. In Proceedings of the 7th ICOM Group on Wet Organic Archaeological Materials Conference, Grenoble, France, 19–23 October 1998; Bonnot-Diconne, C., Hiron, X., Tran, Q.K., Hoffmann, P., Eds.; ICOM-CC: Grenoble, France, 1999; pp. 249–253. [Google Scholar]
- Cook, C.; Grattan, D. A Method of Calculation the Concentration of PEG for Freeze-Drying Waterlogged Wood. In Proceedings of the 4th ICOM-CC Group on Wet Organic Archaeological Materials Conference, Bremerhaven, Germany, 20–24 August 1990; Hoffmann, P., Ed.; ICOM-CC: Bremerhaven, Germany, 1990; pp. 239–252. [Google Scholar]
- Hoffmann, P.; Riens, R.; Eckstein, D. Zur Gefriertrocknung schwer zu konservierender Naßhölzer. Arbeitsblätter der Restauratoren 1991, 13, 193–205. [Google Scholar]
- Penttilä, P.A.; Altgen, M.; Awais, M.; Österberg, M.; Rautkari, L.; Schweins, R. Bundling of Cellulose Microfibrils in Native and Polyethylene Glycol-Containing Wood Cell Walls Revealed by Small-Angle Neutron Scattering. Sci. Rep. 2020, 10, 20844. [Google Scholar] [CrossRef]
- Bardet, M.; Gerbaud, G.; Giffard, M.; Doan, C.; Hediger, S.; Le Pape, L. 13C High-Resolution Solid-State NMR for Structural Elucidation of Archaeological Woods. Prog. Nucl. Magn. Reson. Spectrosc. 2009, 55, 199–214. [Google Scholar] [CrossRef]
- Hoffmann, P. On the Stabilization of Waterlogged Oakwood with PEG. II. Designing a Two-Step Treatment for Multi-Quality Timbers. Stud. Conserv. 1986, 31, 103–113. [Google Scholar] [CrossRef]
- Thill, A.; Spalla, O. Aggregation Due to Capillary Forces during Drying of Particle Submonolayers. Colloids Surf. Physicochem. Eng. Asp. 2003, 217, 143–151. [Google Scholar] [CrossRef]
- Stelzner, I. Transfer into Praxis. Evaluation of Consolidants for Freeze-Drying Archaeological Wood. In Proceedings of the 13th ICOM-CC Group on Wet Organic Archaeological Materials Conference, Florence, Italy, 16–20 May 2016; Williams, E., Hocker, E., Eds.; ICOM-CC: Florence, Italy, 2018; pp. 325–332. [Google Scholar]
- Jensen, P.; Jørgensen, G.; Schnell, U. Dynamic LV-SEM Analyses of Freeze Drying Processes for Waterlogged Wood. In Proceedings of the 8th ICOM Group on Wet Organic Archaeological Materials Conference, Stockholm, Sweden, 11–15 June 2001; Hoffmann, P., Grant, T., Spriggs, J.A., Cook, C., Recht, A., Eds.; ICOM-CC: Bremerhaven, Germany, 2002; pp. 319–333. [Google Scholar]
- Stelzner, I. Zur Nassholzkonservierung Bestimmung Prozessrelevanter Eigenschaften für Die Gefriertrocknung; Staatliche Akademie der Bildenden Künste: Stuttgart, Germany, 2017. [Google Scholar]
- Hoffmann, P. Sucrose for Waterlogged Wood—Not so Simple at All. In Proceedings of the ICOM Committee for Conservation 11th Triennial Meeting Edinburgh, Scotland, 1–6 September 1996; ICOM-CC: Paris, France, 1996; pp. 657–662. [Google Scholar]
- Hoffmann, P.; Perez de Andres, C.; Sierra Mendes, J.L.; Ramiere, R.; Tran, Q.K.; Weber, U.M. European Inter-Laboratory Study on the Conservation of Waterlogged Wood with Sucrose. In Proceedings of the 5th ICOM Group on Wet Organic Archaeological Materials Conference, Portland, Maine, 16–20 August 1993; Hoffmann, P., Daley, T.W., Grant, T., Eds.; ICOM-CC: Bremerhaven, Germany, 1994; pp. 309–335. [Google Scholar]
- Hoffmann, P. Sucrose for Stabilizing Waterlogged Wood. 2 Stabilization and the Degree of Degradation. In Proceedings of the 5th ICOM Group on Wet Organic Archaeological Materials Conference, Portland, Maine, 16–20 August 1993; Hoffmann, P., Daley, T.W., Grant, T., Eds.; ICOM-CC: Bremerhaven, Germany, 1995; pp. 357–379. [Google Scholar]
- Schmitt, U.; Noldt, U. Scanning Electron Microscopic Observations on Saccharose Impregnation of Waterlogged Archaeological Wood; Hoffmann, P., Ed.; ICOM-CC: Bremerhaven, Germany, 1994; pp. 381–390. [Google Scholar]
- Broda, M.; Spear, M.J.; Curling, S.F.; Ormondroyd, G.A. The Viscoelastic Behaviour of Waterlogged Archaeological Wood Treated with Methyltrimethoxysilane. Materials 2021, 14, 5150. [Google Scholar] [CrossRef] [PubMed]
- Popescu, C.-M.; Broda, M. Interactions between Different Organosilicons and Archaeological Waterlogged Wood Evaluated by Infrared Spectroscopy. Forests 2021, 12, 268. [Google Scholar] [CrossRef]
- Broda, M.; Curling, S.F.; Spear, M.J.; Hill, C.A.S. Effect of Methyltrimethoxysilane Impregnation on the Cell Wall Porosity and Water Vapour Sorption of Archaeological Waterlogged Oak. Wood Sci. Technol. 2019, 53, 703–726. [Google Scholar] [CrossRef] [Green Version]
- Grattan, D.W. A Practical Comparative Study of Several Treatments for Waterlogged Wood. Stud. Conserv. 1982, 27, 124–136. [Google Scholar] [CrossRef]
- Imazu, S.; Morgos, A. Conserving Waterlogged Wood Using Sugar Alcohols and Comparison the Effectiveness of Lactitol MC, Saccharose and PEG 4000 Treatment. In Proceedings of the 6th ICOM Group on Wet Organic Archaeological Materials Conference, York, UK, 9–13 September 1996; Hoffmann, P., Daley, T., Grant, T., Spriggs, J.A., Eds.; ICOM-CC: Bremerhaven, Germany, 1997; pp. 235–255. [Google Scholar]
- Imazu, S.; Morgos, A. Lactitol Conservation of a 6 m Long Waterlogged Timber Coffin. In Proceedings of the 7th ICOM-CC Group on Wet Organic Archaeological Materials Conference, Grenoble, France, 19–23 October 1998; Bonnot-Diconne, C., Hiron, X., Tran, K., Hoffmann, P., Eds.; ICOM-CC: Grenoble, France, 1999; pp. 210–214. [Google Scholar]
- Stamm, A.J. Effect of Polyethylene Glycol on The Dimensional Stability of Wood. For. Prod. J. 1959, 9, 375–381. [Google Scholar]
- Janeček, E.-R.; Walsh-Korb, Z.; Bargigia, I.; Farina, A.; Ramage, M.H.; D’Andrea, C.; Nevin, A.; Pifferi, A.; Scherman, O.A. Time-Resolved Laser Spectroscopy for the in Situ Characterization of Methacrylate Monomer Flow within Spruce. Wood Sci. Technol. 2017, 51, 227–242. [Google Scholar] [CrossRef]










| Contemporary Pine Sample | Archaeological Pine Sample | |
|---|---|---|
| Basic density (BD) (kg/m³) | 420 | 251 |
| Cellulose (%) | 49 | 27 |
| Lignin (%) | 28 | 53 |
| Hemicellulose (%) | 23 | 20 |
| Maximum water content MWC (%) | 26–180 1 | 332 |
| Residual basic density (%) | - | 60 |
| Holocellulose, lignin ratio (H/L) | 2.6 | 0.7 |
| Sample No. | Sample No. | Volume Shrinkage (%) | Anti-Shrink Efficiency (%) |
|---|---|---|---|
| Pi1-Air | V03-01 | 23.07 | 0 |
| Pi1-AlEt | V03-17 | 5.65 | 76 |
| Pi1-K800 | V03-41 | 3.06 | 87 |
| Pi1-LaTr | V03-28 | 2.63 | 89 |
| Pi1-PEG1 | V03-35 | 2.03 | 91 |
| Pi1-PEG2 | V03-32 | 3.05 | 87 |
| Pi1-PEG3 | V03-20 | 3.37 | 85 |
| Pi1-Sac | V03-45 | 2.38 | 90 |
| Pi1-Sil | V03-42 | 5.67 | 75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stelzner, I.; Stelzner, J.; Gwerder, D.; Martinez-Garcia, J.; Schuetz, P. Imaging and Assessment of the Microstructure of Conserved Archaeological Pine. Forests 2023, 14, 211. https://doi.org/10.3390/f14020211
Stelzner I, Stelzner J, Gwerder D, Martinez-Garcia J, Schuetz P. Imaging and Assessment of the Microstructure of Conserved Archaeological Pine. Forests. 2023; 14(2):211. https://doi.org/10.3390/f14020211
Chicago/Turabian StyleStelzner, Ingrid, Jörg Stelzner, Damian Gwerder, Jorge Martinez-Garcia, and Philipp Schuetz. 2023. "Imaging and Assessment of the Microstructure of Conserved Archaeological Pine" Forests 14, no. 2: 211. https://doi.org/10.3390/f14020211