Restoration Trajectories and Ecological Thresholds during Planted Urban Forest Successional Development
Abstract
:1. Introduction
1.1. Urban Forest Restoration
1.2. Successional Development and Restoration Trajectories in the Urban Ecosystem Context
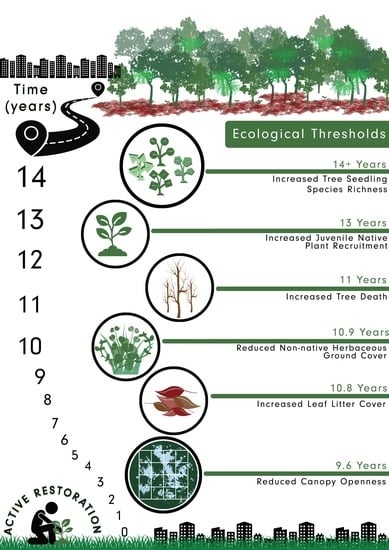
1.3. Ecological Thresholds and Indicators in Restoration
2. Materials and Methods
2.1. Study Site
2.2. Data Collection
2.3. Data Analysis
3. Results
3.1. Canopy Changes
3.2. Ground Covers and Dead Trees
3.3. Native Plant Regeneration in the Understory
3.3.1. Tree Seedlings
3.3.2. Saplings and Epiphytes
4. Discussion
4.1. Ecological Trajectories of Key Ecosystem Attributes
4.2. Ecological Thresholds and Suitable Indicators in Urban Forests
4.3. Implications for Management of Urban Forests Undergoing Restoration
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grimm, N.B.; Faeth, S.H.; Golubiewski, N.E.; Redman, C.L.; Wu, J.; Bai, X.; Briggs, J.M. Global Change and the Ecology of Cities. Science 2008, 319, 756–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Endreny, T.; Santagata, R.; Perna, A.; De Stefano, C.; Rallo, R.; Ulgiati, S. Implementing and managing urban forests: A much needed conservation strategy to increase ecosystem services and urban wellbeing. Ecol. Model. 2017, 360, 328–335. [Google Scholar] [CrossRef]
- Piana, M.R.; Aronson, M.F.; Pickett, S.T.; Handel, S.N. Plants in the city: Understanding recruitment dynamics in urban landscapes. Front. Ecol. Environ. 2019, 17, 455–463. [Google Scholar] [CrossRef]
- Nowak, D.J.; Crane, D.E. Carbon storage and sequestration by urban trees in the USA. Environ. Pollut. 2002, 116, 381–389. [Google Scholar] [CrossRef]
- Nowak, D.J.; Crane, D.E.; Stevens, J.C. Air pollution removal by urban trees and shrubs in the United States. Urban For. Urban Green. 2006, 4, 115–123. [Google Scholar] [CrossRef]
- Xiao, Q.; McPherson, E.G. Rainfall interception by Santa Monica’s municipal urban forest. Urban Ecosyst. 2002, 6, 291–302. [Google Scholar] [CrossRef]
- Dobbs, C.; Escobedo, F.J.; Zipperer, W.C. A framework for developing urban forest ecosystem services and goods indicators. Landsc. Urban Plan. 2011, 99, 196–206. [Google Scholar] [CrossRef]
- Bowler, D.E.; Buyung-Ali, L.; Knight, T.M.; Pullin, A.S. Urban greening to cool towns and cities: A systematic review of the empirical evidence. Landsc. Urban Plan. 2010, 97, 147–155. [Google Scholar] [CrossRef]
- Aronson, M.F.J.; La Sorte, F.A.; Nilon, C.H.; Katti, M.; Goddard, M.A.; Lepczyk, C.A.; Warren, P.S.; Williams, N.S.G.; Clilliers, S.; Clarkson, B.; et al. A global analysis of the impacts of urbanization on bird and plant diversity reveals key anthropogenic drivers. Proc. R. Soc. B Biol. Sci. 2014, 281, 20133330. [Google Scholar] [CrossRef]
- Threlfall, C.G.; Williams, N.; Hahs, A.K.; Livesley, S. Approaches to urban vegetation management and the impacts on urban bird and bat assemblages. Landsc. Urban Plan. 2016, 153, 28–39. [Google Scholar] [CrossRef]
- Brown, G.; Schebella, M.; Weber, D. Using participatory GIS to measure physical activity and urban park benefits. Landsc. Urban Plan. 2014, 121, 34–44. [Google Scholar] [CrossRef]
- Breed, M.F.; Cross, A.T.; Wallace, K.; Bradby, K.; Flies, E.; Goodwin, N.; Jones, M.; Orlando, L.; Skelly, C.; Weinstein, P.; et al. Ecosystem Restoration: A Public Health Intervention. EcoHealth 2020, 18, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Soanes, K.; Lentini, P. When cities are the last chance for saving species. Front. Ecol. Environ. 2019, 17, 225–231. [Google Scholar] [CrossRef]
- Elmqvist, T.; Setälä, H.; Handel, S.N.; van der Ploeg, S.; Aronson, J.; Blignaut, J.N.; Gómez-Baggethun, E.; Nowak, D.J.; Kronenberg, J.; de Groot, R. Benefits of restoring ecosystem services in urban areas. Curr. Opin. Environ. Sustain. 2015, 14, 101–108. [Google Scholar] [CrossRef] [Green Version]
- Standish, R.J.; Hobbs, R.J.; Miller, J.R. Improving city life: Options for ecological restoration in urban landscapes and how these might influence interactions between people and nature. Landsc. Ecol. 2013, 28, 1213–1221. [Google Scholar] [CrossRef] [Green Version]
- Mata, L.; Ramalho, C.E.; Kennedy, J.; Parris, K.M.; Valentine, L.; Miller, M.; Bekessy, S.; Hurley, S.; Cumpston, Z. Bringing nature back into cities. People Nat. 2020, 2, 350–368. [Google Scholar] [CrossRef]
- Piana, M.R.; Hallett, R.A.; Aronson, M.F.J.; Conway, E.; Handel, S.N. Natural regeneration in urban forests is limited by early-establishment dynamics: Implications for management. Ecol. Appl. 2021, 31, e2255. [Google Scholar] [CrossRef]
- Wallace, K.J.; Clarkson, B.D. Urban forest restoration ecology: A review from Hamilton, New Zealand. J. R. Soc. N. Z. 2019, 49, 347–369. [Google Scholar] [CrossRef]
- Johnson, L.R.; Handel, S.N. Restoration treatments in urban park forests drive long-term changes in vegetation trajectories. Ecol. Appl. 2016, 26, 940–956. [Google Scholar] [CrossRef]
- Zipper, S.C.; Schatz, J.; Singh, A.; Kucharik, C.J.; Townsend, P.A.; Ii, S.P.L. Urban heat island impacts on plant phenology: Intra-urban variability and response to land cover. Environ. Res. Lett. 2016, 11, 054023. [Google Scholar] [CrossRef]
- Wallace, K.J.; Laughlin, D.C.; Clarkson, B.D. Exotic weeds and fluctuating microclimate can constrain native plant regeneration in urban forest restoration. Ecol. Appl. 2017, 27, 1268–1279. [Google Scholar] [CrossRef] [PubMed]
- Drinnan, I.N. The search for fragmentation thresholds in a Southern Sydney Suburb. Biol. Conserv. 2005, 124, 339–349. [Google Scholar] [CrossRef]
- Clarkson, B.D.; Wehi, P.M.; Brabyn, L.K. A spatial analysis of indigenous cover patterns and implications for ecological restoration in urban centres, New Zealand. Urban Ecosyst. 2007, 10, 441–457. [Google Scholar] [CrossRef]
- McIntyre, N.E. Ecology of Urban Arthropods: A Review and a Call to Action. Ann. Èntomol. Soc. Am. 2000, 93, 825–835. [Google Scholar] [CrossRef]
- Farnworth, B.; Innes, J.; Kelly, C.; Littler, R.; Waas, J.R. Photons and foraging: Artificial light at night generates avoidance behaviour in male, but not female, New Zealand weta. Environ. Pollut. 2018, 236, 82–90. [Google Scholar] [CrossRef]
- Trammell, T.L.E.; Ralston, H.A.; Scroggins, S.A.; Carreiro, M.M. Foliar production and decomposition rates in urban forests invaded by the exotic invasive shrub, Lonicera maackii. Biol. Invasions 2011, 14, 529–545. [Google Scholar] [CrossRef]
- La Sorte, F.A.; Aronson, M.; Williams, N.; Celesti, L.; Cilliers, S.; Clarkson, B.D.; Dolan, R.W.; Hipp, A.; Klotz, S.; Kühn, I.; et al. Beta diversity of urban floras among European and non-European cities. Glob. Ecol. Biogeogr. 2014, 23, 769–779. [Google Scholar] [CrossRef]
- Morgan, D.K.J.; Waas, J.R.; Innes, J. An inventory of mammalian pests in a New Zealand city. N. Z. J. Zool. 2009, 36, 23–33. [Google Scholar] [CrossRef]
- Hobbs, R.J.; Norton, D.A. Towards a Conceptual Framework for Restoration Ecology. Restor. Ecol. 1996, 4, 93–110. [Google Scholar] [CrossRef]
- Suding, K.N.; Gross, K.L.; Houseman, G.R. Alternative states and positive feedbacks in restoration ecology. Trends Ecol. Evol. 2004, 19, 46–53. [Google Scholar] [CrossRef]
- Odum, E.P. The Strategy of Ecosystem Development. Science 1969, 164, 262–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connell, J.H.; Slatyer, R.O. Mechanisms of Succession in Natural Communities and Their Role in Community Stability and Organization. Am. Nat. 1977, 111, 1119–1144. [Google Scholar] [CrossRef]
- Pickett, S.T.A.; Collins, S.; Armesto, J.J. Models, mechanisms and pathways of succession. Bot. Rev. 1987, 53, 335–371. [Google Scholar] [CrossRef]
- Hobbs, R.J.; Walker, L.R.; Walker, J. Integrating Restoration and Succession. In Linking Restoration and Ecological Succession; Walker, L.R., Walker, J., Hobbs, R.J., Eds.; Springer: New York, NY, USA, 2007; pp. 168–179. [Google Scholar]
- Schick, A.; Porembski, S.; Hobson, P.R.; Ibisch, P.L. Classification of key ecological attributes and stresses of biodiversity for ecosystem-based conservation assessments and management. Ecol. Complex. 2019, 38, 98–111. [Google Scholar] [CrossRef]
- Timpane-Padgham, B.L.; Beechie, T.; Klinger, T. A systematic review of ecological attributes that confer resilience to climate change in environmental restoration. PLoS ONE 2017, 12, e0173812. [Google Scholar] [CrossRef] [Green Version]
- Suding, K.N.; Gross, K.L. The dynamic nature of ecological systems: Multiple states and restoration trajectories. In Foundations of Restoration Ecology; Falk, D.A., Palmer, M., Zedler, J., Eds.; Island Press: Washington, DC, USA, 2006; pp. 190–209. [Google Scholar]
- De Cáceres, M.; Coll, L.; Legendre, P.; Allen, R.B.; Wiser, S.K.; Fortin, M.; Condit, R.; Hubbell, S. Trajectory analysis in community ecology. Ecol. Monogr. 2019, 89, 1350. [Google Scholar] [CrossRef] [Green Version]
- Groffman, P.M.; Baron, J.S.; Blett, T.; Gold, A.J.; Goodman, I.; Gunderson, L.H.; Levinson, B.M.; Palmer, M.A.; Paerl, H.W.; Peterson, G.; et al. Ecological Thresholds: The Key to Successful Environmental Management or an Important Concept with No Practical Application? Ecosystems 2006, 9, 1–13. [Google Scholar] [CrossRef]
- Hobbs, R.J.; Norton, D.A. Ecological filters, thresholds, and gradients in resistance to ecosystem assembly. In Assembly Rules and Restoration Ecology; Island Press: Washington, DC, USA, 2004; pp. 72–94. [Google Scholar]
- Suding, K.N.; Hobbs, R. Threshold models in restoration and conservation: A developing framework. Trends Ecol. Evol. 2009, 24, 271–279. [Google Scholar] [CrossRef]
- Dale, V.H.; Beyeler, S.C. Challenges in the development and use of ecological indicators. Ecol. Indic. 2001, 1, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Gatica-Saavedra, P.; Echeverría, C.; Nelson, C.R. Ecological indicators for assessing ecological success of forest restoration: A world review. Restor. Ecol. 2017, 25, 850–857. [Google Scholar] [CrossRef]
- Siddig, A.A.; Ellison, A.; Ochs, A.; Villar-Leeman, C.; Lau, M.K. How do ecologists select and use indicator species to monitor ecological change? Insights from 14 years of publication in Ecological Indicators. Ecol. Indic. 2016, 60, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Walker, L.R.; Walker, J.; del Moral, R. Forging a new alliance between succession and restoration. In Linking Restoration and Ecological Succession; Walker, L.R., Walker, J., Hobbs, R.J., Eds.; Springer: New York, NY, USA, 2007; pp. 1–18. [Google Scholar]
- Ruiz-Jaen, M.C.; Aide, M.T. Restoration success: How is it being measured? Restor. Ecol. 2005, 13, 569–577. [Google Scholar] [CrossRef]
- Swanson, M.E.; Franklin, J.F.; Beschta, R.L.; Crisafulli, C.M.; DellaSala, D.A.; Hutto, R.L.; Lindenmayer, D.B.; Swanson, F.J. The forgotten stage of forest succession: Early-successional ecosystems on forest sites. Front. Ecol. Environ. 2011, 9, 117–125. [Google Scholar] [CrossRef] [Green Version]
- Laughlin, D.C.; Clarkson, B.D. Tree seedling survival depends on canopy age, cover and initial composition: Trade-offs in forest restoration enrichment planting. Ecol. Restor. 2018, 36, 52–61. [Google Scholar] [CrossRef]
- Lorenz, C.; Van Dijk, G.M.; Van Hattum, A.G.M.; Cofino, W.P. Concepts in river ecology: Implications for indicator development. Rivers Res. Manag. 1997, 13, 501–516. [Google Scholar] [CrossRef]
- Gann, G.D.; McDonald, T.; Walder, B.; Aronson, J.; Nelson, C.R.; Jonson, J.; Hallett, J.G.; Eisenberg, C.; Guariguata, M.R.; Liu, J.; et al. International principles and standards for the practice of ecological restoration. Second edition. Restor. Ecol. 2019, 27, S1–S46. [Google Scholar] [CrossRef] [Green Version]
- Wortley, L.; Hero, J.-M.; Howes, M. Evaluating Ecological Restoration Success: A Review of the Literature. Restor. Ecol. 2013, 21, 537–543. [Google Scholar] [CrossRef]
- Noss, R.F. Indicators for Monitoring Biodiversity: A Hierarchical Approach. Conserv. Biol. 1990, 4, 355–364. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Margules, C.R.; Botkin, D. Indicators of forest sustainability biodiversity: The selection of forest indicator species. Conserv. Biol. 2000, 14, 941–950. [Google Scholar] [CrossRef]
- DeLuca, T.H.; Aplet, G.H.; Wilmer, B.; Burchfield, J. The unknown trajectory of forest restoration: A call for ecosystem monitoring. J. For. 2010, 108, 288–295. [Google Scholar]
- Reid, J.L.; Chaves-Fallas, J.M.; Holl, K.D.; Zahawi, R.A. Tropical forest restoration enriches vascular epiphyte recovery. Appl. Veg. Sci. 2016, 19, 508–517. [Google Scholar] [CrossRef]
- Cortina, J.; Amat, B.; Castillo, V.; Fuentes, D.; Maestre, F.; Padilla, F.; Rojo, L. The restoration of vegetation cover in the semi-arid Iberian southeast. J. Arid Environ. 2011, 75, 1377–1384. [Google Scholar] [CrossRef]
- Clarkson, B.D.; Wehi, P.M.; Brabyn, L.K. Bringing Back Nature into Cities: Urban Land Environments, Indigenous Cover, and Urban Restoration; University of Waikato: Hamilton, New Zealand, 2007. [Google Scholar]
- Clarkson, B.D.; Bryan, C.; Clarkson, F. Reconstructing Hamilton’s Indigenous Ecosystems: The Waiwhakareke Natural Heritage Park; City Green: Singapore, 2012; Volume 4, pp. 60–67. [Google Scholar]
- CBER. Waiwhakareke Natural Heritage Park Operative Management Plan; Hamilton City Council: Hamilton, New Zealand, 2011. [Google Scholar]
- Walker, L.R.; Wardle, D.A.; Bardgett, R.D.; Clarkson, B.D. The use of chronosequences in studies of ecological succession and soil development. J. Ecol. 2010, 98, 725–736. [Google Scholar] [CrossRef]
- Kirby, C.L. Field Guide to New Zealand’s Epiphytes, Vines & Mistletoes; University of Waikato: Hamilton, New Zealand, 2014. [Google Scholar]
- Suganuma, M.S.; Durigan, G. Indicators of restoration success in riparian tropical forests using multiple reference ecosystems. Restor. Ecol. 2014, 23, 238–251. [Google Scholar] [CrossRef]
- Muggeo, V.M.R.; Sciandra, M.; Tomasello, A.; Calvo, S. Estimating growth charts via nonparametric quantile regression: A practical framework with application in ecology. Environ. Ecol. Stat. 2013, 20, 519–531. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Henry, M.; Stevens, M.H.H.; et al. Vegan:community ecology package. 2015. Computer coding software package in R.
- Oliver, W.R.B. New Zealand epiphytes. J. Ecol. 1930, 18, 1–50. [Google Scholar] [CrossRef]
- Clements, F.E. Plant Succession: An Analysis of the Development of Vegetation; Carnegie Institution of Washington: Washington, DC, USA, 1916. [Google Scholar]
- Copeland, T.E.; Sluis, W.; Howe, H.F. Fire Season and Dominance in an Illinois Tallgrass Prairie Restoration. Restor. Ecol. 2002, 10, 315–323. [Google Scholar] [CrossRef]
- Busing, R.T. Tree mortality, canopy turnover, and woody detritus in old cove forests of the southern appalachians. Ecology 2005, 86, 73–84. [Google Scholar] [CrossRef]
- Guariguata, M.R.; Ostertag, R. Neotropical secondary forest succession: Changes in structural and functional characteristics. For. Ecol. Manag. 2001, 148, 185–206. [Google Scholar] [CrossRef]
- Brown, N. The implications of climate and gap microclimate for seedling growth conditions in a Bornean lowland rain forest. J. Trop. Ecol. 1993, 9, 153–168. [Google Scholar] [CrossRef]
- Lindig-Cisneros, R.; Desmond, J.; Boyer, K.E.; Zedler, J.B. Wetland restoration thresholds: Can a degradation transition be reversed with increased effort? Ecol. Appl. 2003, 13, 193–205. [Google Scholar] [CrossRef]
- Caldeira, M.V.W.; Godinho, T.D.O.; Moreira, F.L.; Campanharo Ítalo, F.; Castro, K.C.; De Mendonça, A.R.; Trazzi, P.A. Litter as an Ecological Indicator of Forest Restoration Processes in a Dense Ombrophylous Lowland Forest. Floresta Ambient. 2019, 26, 1. [Google Scholar] [CrossRef] [Green Version]
- Martinelli, L.; Lins, S.R.M.; dos Santos-Silva, J.C. Fine litterfall in the Brazilian Atlantic Forest. Biotropica 2017, 49, 443–451. [Google Scholar] [CrossRef]
- Yelenik, S.G. Linking dominant Hawaiian tree species to understory development in recovering pastures via impacts on soils and litter. Restor. Ecol. 2016, 25, 42–52. [Google Scholar] [CrossRef]
- Ziter, C.D.; Pedersen, E.J.; Kucharik, C.J.; Turner, M.G. Scale-dependent interactions Oliveirabetween tree canopy cover and impervious surfaces reduce daytime urban heat during summer. Proc. Natl. Acad. Sci. USA 2019, 116, 7575–7580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Overdyck, E.; Clarkson, B.D. Seed rain and soil seed banks limit native regeneration within urban forest restoration plantings in Hamilton City, New Zealand. N. Z. J. Ecol. 2012, 36, 177–190. [Google Scholar]
- Bertacchi, M.I.F.; Amazonas, N.T.; Brancalion, P.H.S.; Brondani, G.E.; de Oliveira, A.C.S.; De Pascoa, M.A.R.; Rodrigues, R.R. Establishment of tree seedlings in the understory of restoration plantations: Natural regeneration and enrichment plantings. Restor. Ecol. 2015, 24, 100–108. [Google Scholar] [CrossRef]
- Oldfield, E.E.; Warren, R.J.; Felson, A.; Bradford, M. FORUM: Challenges and future directions in urban afforestation. J. Appl. Ecol. 2013, 50, 1169–1177. [Google Scholar] [CrossRef]
- Young, T.P.; Petersen, D.A.; Clary, J.J. The ecology of restoration: Historical links, emerging issues and unexplored realms. Ecol. Lett. 2005, 8, 662–673. [Google Scholar] [CrossRef]
- Urza, A.K.; Weisberg, P.J.; Chambers, J.C.; Sullivan, B.W. Shrub facilitation of tree establishment varies with ontogenetic stage across environmental gradients. New Phytol. 2019, 223, 1795–1808. [Google Scholar] [CrossRef]
- Grubb, P.J. The maintenance of species-richness in plant communities: The importance of the regeneration niche. Biol. Rev. 1977, 52, 107–145. [Google Scholar] [CrossRef]




| Term | Definition as Used in this Paper | Related Refs. |
|---|---|---|
| Successional development | Also sometimes known as ‘ecological succession’. Succession theory defines as the process of a change in species structure of an ecological community over time. This results largely from modification of the physical environment by the species present and is somewhat predictable and therefore a process that ecological restoration often seeks to mimic. Here we assume successional development should occur in restoration projects following an anthropogenic disturbance and subsequent restoration action and the goal is to reach a ‘mature’, ‘climax’ or ‘stable’ functioning ecosystem possibly sustained at an ‘equilibrium’. | [29,31,32,33,34] |
| Key ecosystem attribute | Also sometimes known as ‘key ecological attribute’ or ‘community attribute’. These are measurable properties of an ecosystem that are useful in monitoring or otherwise quantitatively assessing function or biodiversity and they are often drivers of successional development. They are helpful characteristics to monitor when assessing progress toward a target state for the ecosystem. | [21,35,36] |
| Restoration trajectory | Also sometimes known as ‘ecological trajectory’. The pathway of development taken by either an entire ecological community or individual ecosystem attribute during the process of restoration. In this paper we use the concept when referring to individual key ecosystem attributes. | [37,38] |
| Ecological threshold | An ecological threshold is the point at which there is an abrupt change in an ecosystem attribute or property, or where small changes in an environmental driver produce large responses in the ecosystem. A threshold can often signal a rapid shift or change from one state or successional stage of the ecosystem to another. | [39,40,41] |
| Ecological indicator | The appearance or occurrence of a specified abiotic or biotic property that signals a condition or state of the wider ecosystem. Ecological indicators may differ widely in their specification, depending on their application, and in this paper we discuss in the context of those that may be of most use during urban forest restoration. | [7,42,43,44] |
| Forest Age (y) | Ground Cover Category (Percent Cover) | ||||
|---|---|---|---|---|---|
| Non-Native Herbaceous Plants * | Leaf Litter * | Bare Ground * | Moss | Fern | |
| 1 | 100 | 0 | 0 | 0 | 0 |
| 2 | 100 | 0 | 0 | 0 | 0 |
| 5 | 75 | 20 | 5 | 0 | 0 |
| 6 | 85 | 10 | 5 | 0 | 0 |
| 6 | 70 | 28 | 2 | 0 | 0 |
| 6 | 70 | 20 | 10 | 0 | 0 |
| 7 | 1 | 38 | 60 | 1 | 0 |
| 9 | 70 | 20 | 1 | 1 | 8 |
| 9 | 7 | 89 | 2 | 1 | 1 |
| 9 | 9 | 73 | 15 | 2 | 1 |
| 10 | 25 | 65 | 5 | 1 | 4 |
| 11 | 55 | 25 | 4 | 0 | 16 |
| 11 | 10 | 65 | 5 | 0 | 20 |
| 11 | 0 | 49 | 10 | 1 | 40 |
| 11 | 0 | 50 | 8 | 2 | 40 |
| 11 | 0 | 95 | 5 | 0 | 0 |
| 11 | 1 | 90 | 4 | 0 | 5 |
| 11 | 1 | 88 | 10 | 0 | 1 |
| 11 | 10 | 73 | 15 | 1 | 1 |
| 12 | 70 | 27 | 1 | 1 | 1 |
| 13 | 20 | 10 | 5 | 0 | 65 |
| 13 | 5 | 24 | 5 | 1 | 65 |
| 13 | 79 | 15 | 5 | 0 | 1 |
| 13 | 36.56 | 54.31 | 6 | 3.13 | 0 |
| 14 | 42 | 15 | 1 | 1 | 41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wallace, K.J.; Clarkson, B.D.; Farnworth, B. Restoration Trajectories and Ecological Thresholds during Planted Urban Forest Successional Development. Forests 2022, 13, 199. https://doi.org/10.3390/f13020199
Wallace KJ, Clarkson BD, Farnworth B. Restoration Trajectories and Ecological Thresholds during Planted Urban Forest Successional Development. Forests. 2022; 13(2):199. https://doi.org/10.3390/f13020199
Chicago/Turabian StyleWallace, K. J., Bruce D. Clarkson, and Bridgette Farnworth. 2022. "Restoration Trajectories and Ecological Thresholds during Planted Urban Forest Successional Development" Forests 13, no. 2: 199. https://doi.org/10.3390/f13020199