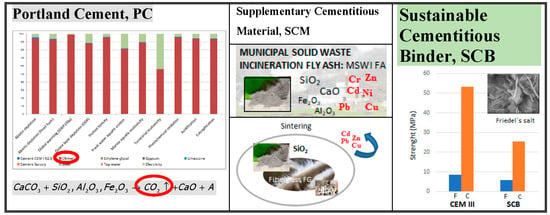
Municipal Solid Waste Incineration Fly Ash: From Waste to Cement Manufacturing Resource
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Methods
2.2.1. Environmental Evaluation of the Cements
Goal and Scope
System Boundaries and Life Cycle Inventory
Data Collection and Impact Assessment Method
2.2.2. Physicochemical Characterization
2.2.3. Study of the Hardened SCBs
2.2.4. Analysis of Heavy Metals
3. Results and Discussion
3.1. Environmental Evaluation
3.2. Physicochemical Characterization
3.3. Hardened SCBs
3.4. Behaviour of Heavy Metals in MSWI FA
4. Conclusions
- The use of MSWI FA-based SCMs could reduce the cement’s GWP by up to 55%.
- The main elements in MSWI FA are calcium, silica, alumina and iron, a composition similar to that of the mineral admixture used in cement-based materials. However, large amounts of chloride and traces of heavy metals are also detected, which means the ashes must be pre-treated before use as SCMs. HT is a useful way to dissolve part of the chloride, while the heavy metals can be eliminated by calcination/vitrification.
- The main minerals present in the SCMs are quartz, gehlenite, portlandite and calcite, the major minerals of raw materials for binders used in construction.
- The presence of vitreous silica improves the mechanical behaviour of the hardened HT MSWI FA-based SCBs, increasing compressive strength by ~30% and flexural strength by ~40%.
- Mortars of HT MSWI FA-based SCBs present a similar microstructure to those of OPC. Thus, hexagonal plates of portlandite, a C-S-H “honeycomb” structure and short fibres of ettringite are generated during the hydration process. When the only SCM in the SCB is HT MSWI FA microcracks are formed that explain the low mechanical strength values of the hardened SCB. These cracks are attributed to the metallic Al components that evolve into hydrogen gas during the curing process. When HT MSWI FA is mixed with calcined and vitrified demolition materials a densification of the mortar takes place and the mechanical behaviour improves.
- TG/DTG, DRX, FTIR and SEM confirmed the presence of Friedel’s salt, a stable aluminate phase whose composition is sensitive to the local chemical environment and with which, in the presence of chloride, an ion exchange takes place. Thus, Friedel’s salt acts as a “sink” for chloride ions and thereby retards diffusion of it.
- The heavy metals in MSWI FA are fixed during the hardening of the SCB.
Recommendations for Further Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AM | aluminate modulus |
| AR | alumina ratio |
| BA | bottom ash |
| CDW | construction and demolition waste |
| CEM I | cement classification of Portland cement without any main addition |
| CEM III/A | commercial blast furnace cement |
| DL | detection limit |
| DTG | derivative function TG curves |
| ELQ | estimated limit of quantification |
| EPD | environmental product declaration |
| FA | fly ash |
| FG | fibreglass |
| FTIR | Fourier transform infrared |
| GWP | global warming potential |
| HM | hydraulic modulus |
| HT | hydrothermal treatment |
| IA | incineration ash |
| ICP-MS | inductively coupled plasma mass spectrometry |
| LCA | life cycle assessment |
| LOI | loss-on-ignition |
| LSF | lime saturation factor |
| MIP | mercury intrusion porosimetry |
| MSW | municipal solid waste |
| MSWI FA | municipal solid waste incineration fly ash |
| MIP | porosity by MIP |
| OPC | Portland cement |
| PCR | product category rules |
| S | blast furnace slag |
| SCB | sustainable cementitious binder |
| SCB1 | the binder with SCM1 |
| SCB2 | the binder with SCM2 |
| SCM | supplementary cementitious material |
| SCM1 | hydrothermally treated MSWI FA |
| SCM2 | MSWI FA with FG from the manufacture of composites and CDW |
| SEM | scanning electron microscope |
| SM | silicate modulus |
| SR | silica modulus |
| TG | thermogravimetric analysis |
| WDXRF | wavelength dispersive X-ray fluorescence |
| XRD | X-ray diffraction |
| XRF | X-ray fluorescence |
References
- Navid, C.; Alireza, F.; Nima, P.; Hadi, F. Experimental Investigation of Sound Transmission Loss in Concrete Containing Recycled Rubber Crumbs. Adv. Concr. Constr. 2021, 11, 447–454. [Google Scholar] [CrossRef]
- Chalangaran, N.; Farzampour, A.; Paslar, N. Nano Silica and Metakaolin Effects on the Behavior of Concrete Containing Rubber Crumbs. CivilEng 2020, 1, 264–274. [Google Scholar] [CrossRef]
- Mansouri, I.; Shahheidari, F.S.; Hashemi, S.M.A.; Farzampour, A. Investigation of steel fiber effects on concrete abrasion resistance. Adv. Concr. Constr. 2020, 9, 367–374. [Google Scholar] [CrossRef]
- Delvoie, S.; Zhao, Z.; Michel, F.; Courard, L. Market analysis of recycled sands and aggregates in North-wets Europe: Drivers and barriers. IOP Conf. Ser. Earth Environ. Sci. 2019, 225, 012055. [Google Scholar] [CrossRef]
- Eberhardt, L.C.M.; Birkved, M.; Birgisdottir, H. Building design and construction strategies for a circular economy. Archit. Eng. Des. Manag. 2022, 18, 93–113. [Google Scholar] [CrossRef]
- Feiz, R.; Ammenberg, J.; Baas, L.; Eklund, M.; Helgstrand, A.; Marshall, R. Improving the CO2 performance of cement, part I: Utilizing life-cycle assessment and key performance indicators to assess development within the cement industry. J. Clean. Prod. 2015, 98, 272–281. [Google Scholar] [CrossRef] [Green Version]
- EIPPCB. Integrated Pollution Prevention and Control (IPPC): Reference Document on Best Available Techniques in the Cement, Lime and Magnesium Oxide Manufacturing Industries; European IPPC Bureau: Seville, Spain, 2010. [Google Scholar]
- Moya, J.A.; Pardo, N.; Mercier, A. Energy Efficiency and CO2 Emissions—Prospective Scenarios for the Cement Industry; JRC-IE Scientific and Technical Reports; European Commission: Petten, The Netherlands, 2010. [Google Scholar] [CrossRef]
- Tait, M.W.; Cheung, W.M. A comparative cradle-to-gate life cycle assessment of three concrete mix designs. Int. J. Life Cycle Assess. 2016, 21, 847–860. [Google Scholar] [CrossRef] [Green Version]
- Silva, R.V.; de Brito, J.; Lynn, C.J.; Dhir, R.K. Environmental impacts of the use of bottom ashes from municipal solid waste incineration: A review. Resour. Conserv. Recycl. 2019, 140, 23–35. [Google Scholar] [CrossRef]
- Mu, Y.; Saffarzadeh, A.; Shimaoka, T. Influence of ignition process on mineral phase transformation in municipal solid waste incineration (MSWI) fly ash: Implications for estimating loss-on-ignition (LOI). Waste Manag. 2017, 59, 222–228. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, Q.; Zhao, Y.; Liu, Y.; Wang, Q.; Khan, M.A.; Chea, Y.; Li, X.; Bai, Y.; Su, X.; et al. Dissolved organic matter (DOM) was detected in MSWI plant: An investigation of DOM and potential toxic elements variation in the bottom ash and fly ash. Sci. Total Environ. 2022, 828, 154339. [Google Scholar] [CrossRef]
- Del Valle-Zermeño, R.; Formosa, J.; Chimenos, J.M.; Martínez, M.; Fernández, A.I. Aggregate material formulated with MSWI bottom ash and APC fly ash for use as secondary building material. Waste Manag. 2013, 33, 621–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunner, P.H.; Rechberger, H. Waste to energy—Key element for sustainable waste management. Waste Manag. 2015, 37, 3–12. [Google Scholar] [CrossRef]
- Phua, Z.; Giannis, A.; Dong, Z.L.; Lisak, G.; Ng, W.J. Characteristics of incineration ash for sustainable treatment and reutilization. Environ. Sci. Pollut. Res. 2019, 26, 16974–16997. [Google Scholar] [CrossRef] [PubMed]
- Polettini, A.; Pomi, R.; Sirini, P.; Testa, F. Properties of Portland cement—Stabilised MSWI fly ashes. J. Hazard. Mater. 2001, 88, 123–138. [Google Scholar] [CrossRef]
- Devahi, P.; Rathod, D.; Muthukkumaran, K. Building material synthetization using municipal solid waste incineration ash: A state-of-the-art review. Environ. Technol. Rev. 2022, 11, 33–48. [Google Scholar] [CrossRef]
- Chimenos, J.M.; Fernández, A.I.; Cervantes, A.; Miralles, L.; Fernández, M.A.; Espiell, F. Optimizing the APC residue washing process to minimize the release of chloride and heavy metals. Waste Manag. 2005, 25, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Fernández Bertos, M.; Li, X.; Simons, S.J.R.; Hills, C.D.; Carey, P.J. Investigation of accelerated carbonation for the stabilization of MSW incinerator ashes and the sequestration of CO2. Green Chem. 2004, 6, 428–436. [Google Scholar] [CrossRef]
- Khatib, J.; Jahami, A.; El Kordi, A.; Sonebi, M.; Malek, Z.; Elchamaa, R.; Dakkour, S. Effect of Municipal Solid Waste Incineration Bottom Ash (MSWI-BA) on the Structural Performance of Reinforced Concrete (RC) Beams. J. Eng. Des. Technol. 2021. [Google Scholar] [CrossRef]
- Ashraf, M.S.; Ghouleh, Z.; Shao, Y. Production of eco-cement exclusively from municipal solid waste incineration residues. Resour. Conserv. Recycl. 2019, 149, 332–342. [Google Scholar] [CrossRef]
- Huang, T.Y.; Chiueh, P.T.; Lo, S.I. Life-cycle environmental and cost impacts of reusing fly ash. Resour. Conserv. Recycl. 2017, 123, 255–260. [Google Scholar] [CrossRef]
- Huang, T.; Zhou, L.; Chen, L.; Liu, W.; Zhang, S.; Liu, L. Mechanism exploration on the aluminium supplementation coupling the electrokinetic-activating geopolymerization that reinforces the solidification of the municipal solid waste incineration fly ashes. Waste Manag. 2020, 103, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Huber, F.; Fellner, J. Integration of life cycle assessment with monetary valuation for resource classification: The case of municipal solid waste incineration fly ash. Resour. Conserv. Recycl. 2018, 139, 17–26. [Google Scholar] [CrossRef]
- Directive of The European Parliament and of The Council Amending Directives 2008/98/EC on Waste, 94/62/EC on Packaging and Packaging Waste, 1999/31/EC on the Landfill of Waste, 2000/53/EC on End-of-Life Vehicles, 2006/66/EC on Batteries and Accumulators and Waste Batteries and Accumulators, and 2012/19/EU on Waste Electrical and Electronic Equipment. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52014PC0397 (accessed on 20 March 2023).
- Goñi, S.; Guerrero, A.; Macías, A. Obtaining cementitious material from municipal solid waste. Mater. Constr. 2007, 57, 41–51. [Google Scholar] [CrossRef]
- Wang, K.S.; Chiang, K.Y.; Perng, J.P.; Sun, C.J. The characteristics study on sintering of municipal solid waste incinerator ashes. J. Hazard. Mater. 1998, 59, 201–210. [Google Scholar] [CrossRef]
- BIO by Deloitte; INERIS. Study to Assess the Impacts of Different Classification Approaches for Hazard Property “HP14”on Selected Waste Streams. 2015. Available online: https://ec.europa.eu/environment/waste/studies/pdf/hazard%20property.pdf (accessed on 20 March 2023).
- Reijnders, I. The cement industry as a scavenger in industrial ecology and the management of hazardous substances. J. Ind. Ecol. 2007, 11, 15–25. [Google Scholar] [CrossRef]
- Yang, Z.; Ji, R.; Liu, L.; Wang, X.; Zhang, Z. Recycling of municipal solid waste incineration by-product for cement composites preparation. Constr. Build. Mater. 2018, 162, 794–801. [Google Scholar] [CrossRef]
- Siddique, R. Utilization of municipal solid waste (MSW) ash in cement and mortar. Resour. Conserv. Recycl. 2010, 54, 1037–1047. [Google Scholar] [CrossRef]
- Shi, D.; Ma, J.; Wang, H.; Wang, P.; Hu, C.; Zhang, J.; Gu, L. Detoxification of PCBs in fly ash from MSW incineration by hydrothermal treatment with composite silicon-aluminium additives and seed induction. Fuel Process. Technol. 2019, 195, 106157. [Google Scholar] [CrossRef]
- Tay, J.H.; Cheong, H.K. Use of ash derived from refuse incineration as a partial replacement of cement. Cem. Concr. Compos. 1991, 13, 171–175. [Google Scholar] [CrossRef]
- Mangialardi, T.; Piga, L.; Schema, G.; Sirini, P. Characteristics of MSW incinerator ash for use in concrete. Environ. Eng. Sci. 1998, 15, 291–297. [Google Scholar] [CrossRef]
- Rémond, S.; Pimienta, P.; Bentz, D.P. Effects of incorporation of municipal solid waste incineration fly ash in cement pastes and mortars. I. Experimental study. Cem. Concr. Res. 2002, 32, 303–311. [Google Scholar] [CrossRef]
- Bie, R.; Chen, P.; Song, X.; Xiaoyu, J. Characteristics of municipal solid waste incineration fly ash with cement solidification treatment. J. Energy Inst. 2016, 98, 704–712. [Google Scholar] [CrossRef]
- Lin, X.; Mao, T.; Chen, J.; Zhang, S.; Li, X.; Yan, J. Thermal cotreatment of municipal solid waste incineration fly ash with sewage sludge: Phase transformation kinetics and fusion characteristics, and heavy metals solidification. J. Clean. Prod. 2021, 317, 128429. [Google Scholar] [CrossRef]
- Colangelo, F.; Cioffi, R.; Montagnaro, F.; Santoro, L. Soluble salt removal from MSWI fly ash and its stabilization for safer disposal and recovery as road basement material. Waste Manag. 2012, 32, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.S.; Chang, F.C.; Shen, Y.H.; Tsai, M.S.; Ko, C.H. Removal of chloride from MSWI fly ash. J. Hazard. Mater. 2012, 237, 116–120. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, Z.; Lin, X.; Wang, F.; Yan, J. Kinetics and fusion characteristics of municipal solid waste incineration fly ash during thermal treatment. Fuel 2020, 279, 118410. [Google Scholar] [CrossRef]
- Zhao, K.; Hu, Y.; Tian, Y.; Chen, D.; Feng, Y. Chlorine removal from MSWI fly ash by thermal treatment: Effects of iron/aluminium additives. J. Environ. Sci. 2020, 88, 112–121. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, J.; Xu, X.; Wang, W.; Deng, Z.; Zhang, Y. Heavy metal stabilization in municipal solid waste incineration fly ash using heavy metal chelating agents. J. Hazard Mater. 2004, 113, 141–146. [Google Scholar] [CrossRef]
- Lan, T.; Meng, Y.; Ju, T.; Song, M.; Chen, Z.; Shen, P.; Du, Y.; Deng, Y.; Han, S.; Jiang, J. Manufacture of alkali-activated and geopolymer hybrid binder (AGHB) by municipal waste incineration fly ash incorporating aluminosilicate supplementary cementitious materials (ASCM). Chemosphere 2022, 303, 134978. [Google Scholar] [CrossRef]
- Marieta, C.; Guerrero, A.; Leon, I. Municipal solid waste incineration fly ash to produce eco-friendly binders for sustainable building construction. Waste Manag. 2021, 120, 114–124. [Google Scholar] [CrossRef]
- Lilkov, V.; Rostovsky, I.; Petrov, O.; Tzvetanova, Y.; Savov, O. Long term study of hardened cement pastes containing silica fume and fly ash. Constr. Build. Mater. 2014, 60, 48–56. [Google Scholar] [CrossRef]
- Li, X.; Chen, Q.; Zhou, Y.; Tyrer, M.; Yu, Y. Stabilization of heavy metals in MSWI fly ash using silica fume. Waste Manag. 2014, 34, 2494–2504. [Google Scholar] [CrossRef] [PubMed]
- Kunther, W.; Ferreiro, S.; Skibsted, J. Influence of the Ca/Si ratio on the compressive strength of cementitious calcium–silicate–hydrate binders. J. Mater. Chem. A 2017, 5, 17401–17412. [Google Scholar] [CrossRef] [Green Version]
- Matanza, A.; Vargas, G.; Leon, I.; Pousse, M.; Salmon, N.; Marieta, C. Life Cycle Analysis of standard and high-performance cements based on carbon nanotubes composites for construction applications. In Proceedings of the World Sustainable Building 2014 Conference, Barcelona, Spain, 28–30 October 2014; Green Building Council España: Madrid, Spain, 2014; pp. 430–437, ISBN 9788469718155. [Google Scholar]
- Valderrama, C.; Ricard Granados, R.; Cortina, J.L.; Gasol, C.M.; Guillem, M.; Josa, A. Implementation of best available techniques in cement manufacturing: A life-cycle assessment study. J. Clean. Prod. 2012, 25, 60–67. [Google Scholar] [CrossRef]
- Proske, T.; Rezvani, M.; Palm, S.; Müller, C.; Graubner, C.A. Concretes made of efficient multi-composite cements with slag and limestone. Cem. Concr. Compos. 2018, 89, 107–119. [Google Scholar] [CrossRef]
- fib Bulletin 67: Guidelines for Green Concrete Structures; International Federation for Structural Concrete-EPFL: Lausanne, Switzerland, 2012; ISSN 1562-3610.
- Rasheeduzzafar, F.; Dakhil, F.H.; Mukarram, K.M. Influence of cement composition and content on the corrosion behaviour of reinforcing steel in concrete. In Concrete Durability: Katherine and Bryant Mather International Conference, Atlanta, GA, USA, 27 April–1 May 1987; American Concrete Institute: Farmington Hills, MI, USA, 1987; pp. 477–502. [Google Scholar]
- Ghorpade, P.A.; Ha, M.-G.; Won-Ho, C.; Park, J.-Y. Combined calcium sulfoaluminate and ordinary Portland cement/Fe(II) system for enhanced dichlorination of trichloroethylene. Chem. Eng. J. 2013, 231, 326–333. [Google Scholar] [CrossRef]
- Goñi, S.; Guerrero, A.; Macías, A.; Lorenzo, M.P. Materias primas alternativas para la fabricación de cementos más ecoeficientes de baja energía. Rev. ALCONPAT 2011, 1, 17–29. [Google Scholar] [CrossRef]
- Guerrero, A.; Fernandez, E.; Macias, A.; Goñi, S. Hydrothermal treatment of fly ash from municipal solid waste incineration. Waste Manag. Ser. 2000, 1, 178–185. [Google Scholar]
- Zhao, C.; Lin, S.; Zhao, Y.; Lin, K.; Tian, L.; Xie, M.; Zhou, T. Comprehensive understanding the transition behaviors and mechanisms of chlorine and metal ions in municipal solid waste incineration fly ash during thermal treatment. Sci. Total Environ. 2022, 807, 150731. [Google Scholar] [CrossRef]
- Binici, H.; Aksogan, O.; Kaplan, H. A study on cement mortars incorporating plain Portland cement (PPC), ground granulated blast-furnace slag (GGBFS) and basaltic pumice. Indian J. Eng. Mater. Sci. 2005, 12, 214–220. [Google Scholar]
- Samen, L.V.E.K.; Yanou, R.N.; Monguen, C.K.F.; Lenwoue, A.R.K.; Tchamba, A.B.; Gnamisi, G.M.T.; Yang, L.; Mempouo, B. Engineering properties of soda-lime glass powder on Portland cement CEM IV-B-L mortar. J. Build. Pathol. Rehabil. 2022, 7, 27–42. [Google Scholar] [CrossRef]
- Shillito, L.; Almond, M.J.; Nicholson, J.; Pantos, M.; Matthews, W. Rapid characterization of archaeological midden components using FT-IR spectroscopy, SEM–EDX and micro-XRD. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 73, 133–139. [Google Scholar] [CrossRef]
- Alberghina, M.F.; Germinario, C.; Bartolozzi, G.; Bracci, S.; Grifa, C.; Izzo, F.; Lubritto, C. The tomb of the diver and the frescoed tombs in Paestum (Southern Italy): New insights from a comparative archaeometric study. PLoS ONE 2020, 15, e0232375. [Google Scholar] [CrossRef] [Green Version]
- Amor, F.; Diouri, A.; Ellouzi, I.; Ouanji, F. Development of Zn-Al-Ti mixed oxides-modified cement phases for surface photocatalytic performance. Case Stud. Constr. Mater. 2018, 9, e00209. [Google Scholar] [CrossRef]
- Horgnies, M.; Chen, J.J.; Bouillon, C. Overview about the use of fourier transform infrared spectroscopy to study cementitious materials. WIT Trans. Eng. Sci. 2013, 77, 251. [Google Scholar] [CrossRef] [Green Version]
- Ylmén, R.; Jäglid, U.; Steenari, B.M.; Panas, I. Early hydration and setting of Portland cement monitored by IR, SEM and Vicat techniques. Cem. Concr. Res. 2009, 39, 433–439. [Google Scholar] [CrossRef]
- Igisu, M.; Ueno, Y.; Takai, K. FTIR microspectroscopy of carbonaceous matter in ~3.5 Ga seafloor hydrothermal deposits in the North Pole area, Western Australia. Prog. Earth Planet. Sci. 2018, 5, 85. [Google Scholar] [CrossRef]
- Suherman, P.M.; van Riessen, A.; O’Connor, B.; Li, D.; Bolton, D.; Fairhurst, H. Determination of amorphous phase levels in Portland cement Clinker. Powder Diffr. 2002, 17, 178–185. [Google Scholar] [CrossRef]
- Franus, W.; Panek, R.; Wdowin, M. SEM Investigation of Microstructures in Hydration Products of Portland Cement. In 2nd International Multidisciplinary Microscopy and Microanalysis Congress, Proceedings of the InterM, Oludeniz, Turkey, 16–19 October 2014; Polychroniadis, E., Oral, A., Ozer, M., Eds.; Springer Proceedings in Physics 164; Springer: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Birnin-Yauri, U.A.; Glasser, F.P. Friedel’s salt, Ca2Al(OH)6(Cl,OH)·2H2O: Its solid solutions and their role in chloride binding. Cem. Concr. Res. 1998, 28, 1713–1723. [Google Scholar] [CrossRef]
- Tracz, T. Open porosity of cement pastes and their gas permeability. Bull. Pol. Acad. Sci. Tech. Sci. 2016, 64, 775–783. [Google Scholar] [CrossRef]
- Alderete, N.; Villagrán, Y.; Mignon, A.; Snoeck, D.; De Belie, N. Pore structure description of mortars containing ground granulated blast-furnace slag by mercury intrusion porosimetry and dynamic vapour sorption. Constr. Build. Mater. 2017, 145, 157–165. [Google Scholar] [CrossRef]
- Yu, Z.; Ye, G. The pore structure of cement paste blended with fly ash. Constr. Build. Mater. 2013, 45, 30–35. [Google Scholar] [CrossRef]
- López-Uceda, A.; Ayuso, J.; López, M.; Jimenez, J.R.; Agrela, F.; Sierra, M.J. Properties of Non-Structural Concrete Made with Mixed Recycled Aggregates and Low Cement Content. Materials 2016, 9, 74. [Google Scholar] [CrossRef] [Green Version]
- Irshidat, M.H.; Al-Nuaimi, N.; Rabie, M. Potential utilization of municipal solid waste incineration ashes as sand replacement for developing sustainable cementitious binder. Constr. Build. Mater. 2021, 312, 125488. [Google Scholar] [CrossRef]
- Pavlik, Z.; Trnik, A.; Kulovana, T.; Scheinherrová, L.; Rahhal, V.; Irassar, E.; Cerny, R. DSC and TG analysis of a blended binder based on waste ceramic powder and Portland cement. Int. J. Thermophys. 2016, 37, 32. [Google Scholar] [CrossRef]
- Yue, Y.; Wang, J.J.; Basheer, P.A.M.; Bai, Y. Raman spectroscopic investigation of Friedel’s salt. Cem. Concr. Compos. 2018, 86, 306–314. [Google Scholar] [CrossRef]
- Sauman, Z. Carbonization of porous concrete and its main binding components. Cem. Concr. Res. 1971, 1, 645–662. [Google Scholar] [CrossRef]
- Diamond, S. The microstructures of cement paste in concrete. In Proceedings of the 8th International Congress on the Chemistry of Cement, Rio de Janeiro, Brazil, 22–27 September 1986; Volume 1, pp. 113–121. [Google Scholar]
- Girskas, G.; Kizinievič, O.; Kizinievič, V. Analysis of durability (frost resistance) of MSWI fly ash modified cement composites. Arch. Civ. Mech. Eng. 2021, 21, 39. [Google Scholar] [CrossRef]
- Bachtiar, E.; Rachim, F.; Makbul, R.; Tata, A.; Urfan-Ul-Hassan, M.; Kırgız, M.S.; Syarif, M.; de Sousa Galdino, A.G.; Khitab, A.; Benjeddou, O.; et al. Monitoring of chloride and Friedel’s salt, hydration components, and porosity in high-performance concrete. Case Stud. Constr. Mater. 2022, 17, e01208. [Google Scholar] [CrossRef]
- Zhang, Y.; Chang, J.; Ji, J. AH3 phase in the hydration product system of AFt-AFm-AH3 in calcium sulfoaluminate cements: A microstructural study. Constr. Build. Mater. 2018, 167, 587–596. [Google Scholar] [CrossRef]
- Łaźniewska-Piekarczyk, B.; Czop, M.; Smyczek, D. The Comparison of the Environmental Impact of Waste Mineral Wool and Mineral in Wool-Based Geopolymer. Materials 2022, 15, 2050. [Google Scholar] [CrossRef] [PubMed]
- Council Directive 91/271/EEC Concerning Urban Waste-Water Treatment. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A31991L0271 (accessed on 20 March 2023).







| Sample | SiO2 | Al2O3 | Fe2O3 * | MnO | MgO | CaO | Na2O | K2O | TiO2 | P2O5 | SO3 | Cl | LOI ** |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K | 19.70 | 5.37 | 2.86 | 0.04 | 1.41 | 61.23 | 0.10 | 0.89 | 0.22 | 0.33 | 2.01 | 0.14 | 1.55 |
| S | 35.47 | 9.42 | 0.28 | 0.11 | 7.56 | 41.82 | 0.01 | 0.45 | 0.69 | 0.00 | 1.75 | 0.10 | 1.11 |
| GF | 50.22 | 12.72 | 0.23 | DL 1 | 0.41 | 20.92 | 0.55 | 0.19 | 0.08 | 0.02 | 0.06 | 0.18 | 5.44 |
| PC | 8.02 | 2.01 | 1.11 | 0.02 | 0.73 | 46.48 | DL | 0.37 | 0.10 | 0.02 | 0.85 | DL | 37.60 |
| CEM III | 21.13 | 6.26 | 2.04 | 0.06 | 2.74 | 53.53 | 0.10 | 0.73 | 0.33 | 0.12 | 3.66 | 0.25 | 5.80 |
| SCM1 | 17.22 | 5.34 | 1.10 | 0.04 | 2.00 | 30.95 | 9.84 | 1.77 | 1.10 | 1.22 | 1.11 | 3.32 | 23.96 |
| SCB1 | 15.74 | 5.40 | 2.15 | 0.05 | 1.84 | 48.47 | 0.93 | 1.26 | 0.62 | 0.78 | 5.07 | 1.98 | 13.94 |
| SCM2 | 33.69 | 9.27 | 1.33 | 0.03 | 1.66 | 40.45 | 0.57 | 0.26 | 0.93 | 0.95 | 3.26 | 0.35 | 3.72 |
| SCB2 | 21.73 | 6.03 | 2.12 | 1.48 | 1.48 | 50.76 | 1.07 | 1.07 | 0.48 | 0.57 | 4.51 | 2.66 | 7.72 |
| Sample | Cr (μg/mL) | Ni (ng/mL) | Cu (ng/mL) | Zn (ng/mL) | Cd (ng/mL) | Pb (ng/mL) |
|---|---|---|---|---|---|---|
| SCM2 | 2.9 | 70.9 | 232.9 | 84.1 | 1.7 | ELQ |
| SCB1 | 0.3 | ELQ | 15.5 | 40.6 | ELQ | ELQ |
| SCB2 | 0.1 | ELQ | ELQ | ELQ | ELQ | ELQ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marieta, C.; Martín-Garin, A.; Leon, I.; Guerrero, A. Municipal Solid Waste Incineration Fly Ash: From Waste to Cement Manufacturing Resource. Materials 2023, 16, 2538. https://doi.org/10.3390/ma16062538
Marieta C, Martín-Garin A, Leon I, Guerrero A. Municipal Solid Waste Incineration Fly Ash: From Waste to Cement Manufacturing Resource. Materials. 2023; 16(6):2538. https://doi.org/10.3390/ma16062538
Chicago/Turabian StyleMarieta, Cristina, Alexander Martín-Garin, Iñigo Leon, and Ana Guerrero. 2023. "Municipal Solid Waste Incineration Fly Ash: From Waste to Cement Manufacturing Resource" Materials 16, no. 6: 2538. https://doi.org/10.3390/ma16062538