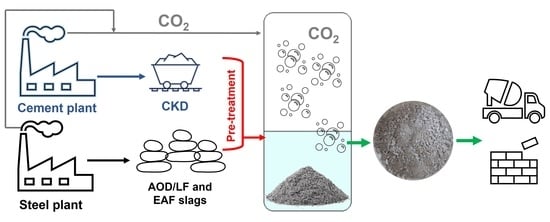
Accelerated Direct Carbonation of Steel Slag and Cement Kiln Dust: An Industrial Symbiosis Strategy Applied in the Bergamo–Brescia Area
Abstract
:1. Introduction
2. Materials and Methods
2.1. Starting Industrial By-Products
2.2. Sample Pre-Treatment
2.3. Characterization
2.3.1. XRD Analysis
2.3.2. XRF Analysis
2.3.3. SEM Analysis
2.3.4. IR Analysis
2.4. Accelerated Carbonation Test
- The Rietveld method with an internal standard was used to calculate the amount of sequestered CO2. This was done by comparing the weight percentage of calcium carbonate before and after carbonation and considering the stoichiometric ratio between CaCO3 and CO2 in the following reactions:
- 2.
- The amount of CO2 sequestered was calculated using the difference between the final mass of the CO2 sequestered and the initial mass of CO2 injected. The system was closed, and the components of the setup, inserted slurry, and CO2 were weighed at each step of the test. The mass of CO2 sequestered was the difference between the final mass of the setup after removing the unreacted CO2 and the initial mass of the setup. If the final mass and the initial mass of the setup corresponded, there was no loss of CO2.
- 3.
- The perfect gas law was applied by monitoring the pressure during the entire process using the initial pressure (pi), final pressure (pf), and initial mass of CO2, as determined via the following equation:
3. Results and Discussion
SEM Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Mineralogical Phases | Chemical Formula | Reference Code | Industrial Waste |
|---|---|---|---|
| Albite | NaAlSi3O8 | 00-009-0466 | CKD |
| Aragonite | CaCO3 | 00-041-1475 | AOD_1, LF_2 |
| Bredigite | Ca1.7Mg0.3SiO4 | 00-035-0260 | AOD_1 |
| Brownmillerite | Ca2(Al,Fe)2O5 | 00-030-0226 | EAF_1 |
| Brucite | Mg(OH)2 | 01-074-2220 | LF_2 |
| Calcite | CaCO3 | 01-080-0941 | AOD_1, LF_2 |
| Calcite | CaCO3 | 00-005-0586 | EAF_1, CKD |
| Calcite | CaCO3 | 01-072-1937 | EAF_2, EAF_3 + SiO2 |
| Calcite monohydrate | CaCO3*H2O | 01-084-0049 | EAF_2 + SiO2 |
| Calcium oxide | CaO | 01-082-1690 | CKD |
| Calcium silicate | Ca2SiO4 | 00-031-0297 | AOD_1 |
| Calcium silicate | Ca2SiO4 | 00-036-0642 | AOD_1 |
| Dolomite | CaMg(CO3)2 | 01-075-1710 | AOD_1 |
| Garnet | Ca1.92Fe3.08O12Si3 | 01-082-1552 | AOD_1 |
| Gehlenite | Ca2Al2SiO7 | 00-020-0199 | AOD_1, LF_2 |
| Gehlenite | Ca2Al2SiO7 | 00-009-0216 | EAF_2, EAF_2 + SiO2 |
| Hydrogarnet | Ca3Al2(H4O4)3 | 01-084-1353 | LF_2 |
| Larnite | Ca2SiO4 | 00-033-0302 | LF_2, EAF_1 |
| Larnite | Ca2SiO4 | 00-029-0369 | EAF_2, EAF_2 + SiO2 |
| Larnite | Ca2SiO4 | 00-020-0237 | EAF_3 + SiO2 |
| Maghemite | Fe2O3 | 00-025-1402 | EAF_1 |
| Magnetite | Fe3O4 | 01-079-0418 | EAF_1, EAF_2, EAF_2 + SiO2 |
| Magnetite | Fe3O4 | 01-072-2303 | EAF_3 + SiO2 |
| Mayenite | Ca12Al14O33 | 00-009-0413 | AOD_1 |
| Olivine | Ca2SiO4 | 01-080-0941 | LF_2 |
| Periclase | MgO | 01-036-0678 | AOD_1 |
| Portlandite | Ca(OH)2 | 00-044-1481 | AOD_1,EAF_1 |
| Quartz | SiO2 | 00-046-1045 | CKD |
| Quartz | SiO2 | 01-079-19-06 | LF_2 |
| Srebrodolskite | Ca2Fe2O5 | 01-074-1860 | EAF_2, EAF_2 + SiO2 |
| Wuestite | FeO | 01-077-2355 | EAF_1 |
| Wuestite | Fe0.974O | 01-073-2143 | LF_2, EAF_2, EAF_2 + SiO2 |
| Yoshiokaite | Ca2(Al,Si)2O4 | 00-046-1336 | EAF_2, EAF_2 + SiO2 |
| AOD_1 | Carbonated AOD_1 | LF_2 | Carbonated LF2 | CKD | Carbonated CKD | ||
|---|---|---|---|---|---|---|---|
| Albite | % | <1 | <1 | ||||
| Garnet | % | 3.9 | 3.6 | ||||
| Hydrogarnet | % | 8.8 | 14.3 | ||||
| Mayenite | % | 12 | 2.8 | ||||
| Bredigite | % | 2.2 | 2.2 | ||||
| Brucite | % | <1 | <1 | ||||
| Dolomite | % | 3.7 | 3 | 1.45 | <1 | ||
| Gehlenite | % | <1 | <1 | 8.1 | 5.4 | ||
| Periclase | % | 5.6 | 5.2 | 8.95 | 5.1 | ||
| Portlandite | % | 1 | < 1 | ||||
| Calcium silicate | % | 20.7 | 12.3 | ||||
| Quartz | % | 1.3 | 2.2 | 6 | 5.6 | ||
| Merwinite | % | 4.6 | 0.5 | ||||
| Wuestite | % | 2.9 | <1 | ||||
| Larnite | % | 13.1 | 8.3 | ||||
| Olivine | % | 15.6 | 17.7 | ||||
| Lime | % | 1.1 | <1 | ||||
| Calcite | % | 2.7 | 16.7 | 3.2 | 8.8 | 86 | 94 |
| Aragonite | % | <1 | 1 | <1 | 8.7 | ||
| Amorphous | % | 49.0 | 54.0 | 31.4 | 29.5 | 6.9 | <1 |
| EAF_1 | Carbonated EAF_1 | EAF_2 | Carbonated EAF_2 | EAF_2 + SiO2 | Carbonated EAF_2 + SiO2 | EAF_3 + SiO2 | Carbonated EAF_3 + SiO2 | ||
|---|---|---|---|---|---|---|---|---|---|
| Browmillerite | % | 7.4 | 9.31 | ||||||
| Portlandite | % | 1 | <1 | ||||||
| Larnite | % | 28.5 | 21.2 | 10.3 | 1.3 | 6.1 | <1 | 7.2 | 6.7 |
| Maghemite | % | 6.9 | 7.8 | ||||||
| Wuestite | % | 11.3 | 11.7 | 18.5 | 9.3 | 9.3 | 7.3 | ||
| Gehlenite | % | 11.5 | 8.8 | 7.4 | 6.2 | ||||
| Magnetite | % | 2.6 | 3.1 | 3 | 5.7 | 3.2 | 2.1 | 9.2 | 16.6 |
| Yoshiokaite | % | <1 | 3.6 | <1 | <1 | ||||
| Srebrodolskite | % | 4.4 | 3.7 | 2.6 | < 1 | ||||
| Calcite | % | <1 | 16.7 | <1 | 7.5 | <1 | 14 | ||
| Monohydrate calcite | % | <1 | 9.2 | ||||||
| Amorphous | % | 41.8 | 29.8 | 52.0 | 60.2 | 71.1 | 73.5 | 82.7 | 62.3 |


References
- Liu, W.; Teng, L.; Rohani, S.; Qin, Z.; Zhao, B.; Xu, C.C.; Ren, S.; Liu, Q.; Liang, B. CO2 Mineral Carbonation Using Industrial Solid Wastes: A Review of Recent Developments. Chem. Eng. J. 2021, 416, 129093. [Google Scholar] [CrossRef]
- Jim Skea, P.R.S. Climate Change 2022, 2022.
- European Union. Direttiva 2009/31/CE del Parlamento europeo e del Consiglio del 23 Aprile 2009; European Union: Brussels, Belgium, 2009. [Google Scholar]
- Yadav, S.; Mehra, A. A Review on Ex Situ Mineral Carbonation. Environ. Sci. Pollut. Res. 2021, 28, 12202–12231. [Google Scholar] [CrossRef]
- Wang, D.; Xiao, J.; Duan, Z. Strategies to Accelerate CO2 Sequestration of Cement-Based Materials and Their Application Prospects. Constr. Build. Mater. 2022, 314, 125646. [Google Scholar] [CrossRef]
- Sorrentino, G.P.; Zanoletti, A.; Ducoli, S.; Zacco, A.; Iora, P.; Invernizzi, C.M.; Di Marcoberardino, G.; Depero, L.E.; Bontempi, E. Accelerated and Natural Carbonation of a Municipal Solid Waste Incineration (MSWI) Fly Ash Mixture: Basic Strategies for Higher Carbon Dioxide Sequestration and Reliable Mass Quantification. Environ. Res. 2023, 217, 114805. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Lewis, J.B.; Klein-BenDavid, O.; Garrabrants, A.C.; Delapp, R.; van der Sloot, H.A.; Kosson, D.S. The Role of Environmental Conditions on the Carbonation of an Alkali-Activated Cementitious Waste Form. Cem. Concr. Res. 2022, 151, 106645. [Google Scholar] [CrossRef]
- Polettini, A.; Pomi, R.; Stramazzo, A. Carbon Sequestration through Accelerated Carbonation of BOF Slag: Influence of Particle Size Characteristics. Chem. Eng. J. 2016, 298, 26–35. [Google Scholar] [CrossRef]
- Pan, S.-Y.; Chang, E.E.; Chiang, P.-C. CO2 Capture by Accelerated Carbonation of Alkaline Wastes: A Review on Its Principles and Applications. Aerosol Air Qual. Res. 2012, 12, 770–791. [Google Scholar] [CrossRef]
- Baciocchi, R.; Costa, G.; Di Gianfilippo, M.; Polettini, A.; Pomi, R.; Stramazzo, A. Thin-Film versus Slurry-Phase Carbonation of Steel Slag: CO2 Uptake and Effects on Mineralogy. J. Hazard. Mater. 2015, 283, 302–313. [Google Scholar] [CrossRef]
- Huntzinger, D.N.; Gierke, J.S.; Kawatra, S.K.; Eisele, T.C.; Sutter, L.L. Carbon Dioxide Sequestration in Cement Kiln Dust through Mineral Carbonation. Environ. Sci. Technol. 2009, 43, 1986–1992. [Google Scholar] [CrossRef]
- Neeraj; Yadav, S. Carbon Storage by Mineral Carbonation and Industrial Applications of CO2. Mater. Sci. Energy Technol. 2020, 3, 494–500. [Google Scholar] [CrossRef]
- Olabi, A.G.; Wilberforce, T.; Elsaid, K.; Sayed, E.T.; Maghrabie, H.M.; Abdelkareem, M.A. Large Scale Application of Carbon Capture to Process Industries—A Review. J. Clean. Prod. 2022, 362, 132300. [Google Scholar] [CrossRef]
- Eurofer. European Steel in Figures 2022; Eurofer: Brussels, Belgium, 2021. [Google Scholar]
- Falsafi, M.; Fornasiero, R. Explorative Multiple-Case Research on the Scrap-Based Steel Slag Value Chain: Opportunities for Circular Economy. Sustainability 2022, 14, 2284. [Google Scholar] [CrossRef]
- Bonenfant, D.; Kharoune, L.; Sauve´, S.; Hausler, R.; Niquette, P.; Mimeault, M.; Kharoune, M. CO2 Sequestration Potential of Steel Slags at Ambient Pressure and Temperature. Ind. Eng. Chem. Res. 2008, 47, 7610–7616. [Google Scholar] [CrossRef]
- Huijgen, W.J.J.; Witkamp, G.-J.; Comans, R.N.J. Mineral CO2 Sequestration by Steel Slag Carbonation. Environ. Sci. Technol. 2005, 39, 9676–9682. [Google Scholar] [CrossRef]
- Huijgen, W.J.J.; Comans, R.N.J. Carbonation of Steel Slag for CO2 Sequestration: Leaching of Products and Reaction Mechanisms. Environ. Sci. Technol. 2006, 40, 2790–2796. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Zhang, Y.; Zhang, M.; Zhao, M.; Wang, Q. Carbonation Curing of Alkaline Industrial Waste for Binders: Comparison of Different Wastes. Mag. Concr. Res. 2022, 74, 143–153. [Google Scholar] [CrossRef]
- Hou, G.; Yan, Z.; Sun, J.; Naguib, H.M.; Lu, B.; Zhang, Z. Microstructure and Mechanical Properties of CO2-Cured Steel Slag Brick in Pilot-Scale. Constr. Build. Mater. 2021, 271, 121581. [Google Scholar] [CrossRef]
- Ortega, I.; Faik, A.; Gil, A.; Rodríguez-Aseguinolaza, J.; D’Aguanno, B. Thermo-Physical Properties of a Steel-Making by-Product to Be Used as Thermal Energy Storage Material in a Packed-Bed System. Energy Procedia 2015, 69, 968–977. [Google Scholar] [CrossRef]
- Gadikota, G.; Park, A.A. Accelerated Carbonation of Ca- and Mg-Bearing Minerals and Industrial Wastes Using CO2. In Carbon: Dioxide Utilisation; Elsevier: Amsterdam, The Netherlands, 2015; pp. 115–137. [Google Scholar]
- Polettini, A.; Pomi, R.; Stramazzo, A. CO2 Sequestration through Aqueous Accelerated Carbonation of BOF Slag: A Factorial Study of Parameters Effects. J. Environ. Manag. 2016, 167, 185–195. [Google Scholar] [CrossRef]
- Song, Q.; Guo, M.-Z.; Wang, L.; Ling, T.-C. Use of Steel Slag as Sustainable Construction Materials: A Review of Accelerated Carbonation Treatment. Resour. Conserv. Recycl. 2021, 173, 105740. [Google Scholar] [CrossRef]
- Al-Bakri, A.Y.; Ahmed, H.M.; Hefni, M.A. Cement Kiln Dust (CKD): Potential Beneficial Applications and Eco-Sustainable Solutions. Sustainability 2022, 14, 7022. [Google Scholar] [CrossRef]
- Li, L.; Ling, T.-C.; Pan, S.-Y. Environmental Benefit Assessment of Steel Slag Utilization and Carbonation: A Systematic Review. Sci. Total Environ. 2022, 806, 150280. [Google Scholar] [CrossRef] [PubMed]
- Anastasiou, E.K.; Liapis, A.; Papachristoforou, M. Life Cycle Assessment of Concrete Products for Special Applications Containing EAF Slag. Procedia Environ. Sci. 2017, 38, 469–476. [Google Scholar] [CrossRef]
- Abdel-Ghani, N.T.; El-Sayed, H.A.; El-Habak, A.A. Utilization of By-Pass Cement Kiln Dust and Air-Cooled Blast-Furnace Steel Slag in the Production of Some “Green” Cement Products. HBRC J. 2018, 14, 408–414. [Google Scholar] [CrossRef]
- Sharma, D.; Goyal, S. Accelerated Carbonation Curing of Cement Mortars Containing Cement Kiln Dust: An Effective Way of CO2 Sequestration and Carbon Footprint Reduction. J. Clean. Prod. 2018, 192, 844–854. [Google Scholar] [CrossRef]
- Huntzinger, D.N.; Gierke, J.S.; Sutter, L.L.; Kawatra, S.K.; Eisele, T.C. Mineral Carbonation for Carbon Sequestration in Cement Kiln Dust from Waste Piles. J. Hazard. Mater. 2009, 168, 31–37. [Google Scholar] [CrossRef]
- Federacciai. La Valorizzazione degli Aggregati di Origine Siderurgica; Federacciai: Milano, Italy, 2012. [Google Scholar]
- Baciocchi, R.; Costa, G.; di Bartolomeo, E.; Polettini, A.; Pomi, R. Carbonation of Stainless Steel Slag as a Process for CO2 Storage and Slag Valorization. Waste Biomass Valoriz. 2010, 1, 467–477. [Google Scholar] [CrossRef]
- Chiang, P.-C.; Pan, S.-Y. Carbon Dioxide Mineralization and Utilization; Springer: Singapore, 2017. [Google Scholar]
- Doebelin, N.; Kleeberg, R. Profex: A Graphical User Interface for the Rietveld Refinement Program BGMN. J. Appl. Cryst. 2015, 48, 1573–1580. [Google Scholar] [CrossRef] [PubMed]
- Assi, A.; Federici, S.; Bilo, F.; Zacco, A.; Depero, L.E.; Bontempi, E. Increased Sustainability of Carbon Dioxide Mineral Sequestration by a Technology Involving Fly Ash Stabilization. Materials 2019, 12, 2714. [Google Scholar] [CrossRef]
- Brand, A.S.; Fanijo, E.O. A Review of the Influence of Steel Furnace Slag Type on the Properties of Cementitious Composites. Appl. Sci. 2020, 10, 8210. [Google Scholar] [CrossRef]
- Alanyalı, H.; Çöl, M.; Yılmaz, M.; Karagöz, Ş. Application of Magnetic Separation to Steelmaking Slags for Reclamation. Waste Manag. 2006, 26, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Abdelzaher, M.; Hamouda, A.; El-Kattan, I.; Baher, A. Laboratory Study for Accelerating the CKD Mineral Carbonation. Egypt. J. Chem. 2022, 65, 491–499. [Google Scholar] [CrossRef]
- Capeletti, L.B.; Zimnoch, J.H. Fourier Transform Infrared and Raman Characterization of Silica-Based Materials. In Applications of Molecular Spectroscopy to Current Research in the Chemical and Biological Sciences; InTech: Rijeka, Croatia, 2016. [Google Scholar]
- Baciocchi, R.; Costa, G.; Polettini, A.; Pomi, R. Influence of Particle Size on the Carbonation of Stainless Steel Slag for CO2 Storage. Energy Procedia 2009, 1, 4859–4866. [Google Scholar] [CrossRef]
- Xu, B.; Yi, Y. Soft Clay Stabilization Using Ladle Slag-Ground Granulated Blastfurnace Slag Blend. Appl. Clay Sci. 2019, 178, 105136. [Google Scholar] [CrossRef]
- Chai, Y.E.; Miller, Q.R.S.; Schaef, H.T.; Barpaga, D.; Bakhshoodeh, R.; Bodor, M.; Van Gerven, T.; Santos, R.M. Pressurized in Situ X-Ray Diffraction Insights into Super/Subcritical Carbonation Reaction Pathways of Steelmaking Slags and Constituent Silicate Minerals. J. Supercrit. Fluids 2021, 171, 105191. [Google Scholar] [CrossRef]
- Bodor, M.; Santos, R.M.; Kriskova, L.; Elsen, J.; Vlad, M.; Van Gerven, T. Susceptibility of Mineral Phases of Steel Slags towards Carbonation: Mineralogical, Morphological and Chemical Assessment. Eur. J. Mineral. 2013, 25, 533–549. [Google Scholar] [CrossRef]
- Johnson, D.C.; Macleod, C.L.; Carey, P.J.; Hills, C.D. Solidification of Stainless Steel Slag by Accelerated Carbonation. Environ. Technol. 2003, 23, 671. [Google Scholar] [CrossRef]
- Jang, J.-H.; Dempsey, B.A.; Burgos, W.D. Solubility of Hematite Revisited: Effects of Hydration. Environ. Sci. Technol. 2007, 41, 7303–7308. [Google Scholar] [CrossRef]
- Baciocchi, R.; Costa, G.; Zingaretti, D.; Cazzotti, M.; Werner, M.; Polettini, A.; Pomi, R.; Falasca, M. Studio Sulle Potenzialità della Carbonatazione di Minerali e Residui Industriali per lo Stoccaggio di Anidride Carbonica Prodotta da Impianti di Piccola/Media Taglia; ENEA: Rome, Italy, 2010. [Google Scholar]
- Alexander, G.B.; Heston, W.M.; Iler, R.K. The Solubility of Amorphous Silica in Water. J. Phys. Chem. 1954, 58, 453–455. [Google Scholar] [CrossRef]
- Yu, P.; Kirkpatrick, R.J.; Poe, B.; McMillan, P.F.; Cong, X. Structure of Calcium Silicate Hydrate (C-S-H): Near-, Mid-, and Far-Infrared Spectroscopy. J. Am. Ceram. Soc. 2004, 82, 742–748. [Google Scholar] [CrossRef]
- Pan, S.-Y.; Chen, Y.-H.; Fan, L.-S.; Kim, H.; Gao, X.; Ling, T.-C.; Chiang, P.-C.; Pei, S.-L.; Gu, G. CO2 Mineralization and Utilization by Alkaline Solid Wastes for Potential Carbon Reduction. Nat. Sustain. 2020, 3, 399–405. [Google Scholar] [CrossRef]
- Gunning, P.J.; Hills, C.D.; Carey, P.J. Accelerated Carbonation Treatment of Industrial Wastes. Waste Manag. 2010, 30, 1081–1090. [Google Scholar] [CrossRef]
- Pedraza, J.I.; Suarez, L.A.; Martinez, L.A.; Rojas, N.Y.; Tobon, J.I.; Ramirez, J.H.; Zea, H.R.; Caceres, A.A. Carbon Capture and Utilization by Mineral Carbonation with CKD in Aqueous Phase: Experimental Stage and Characterization of Carbonated Products. In Proceedings of the 7th International Workshop, Advances in Cleaner Production, Academic Work, Barranquilla, Colombia, 21–22 June 2018; pp. 21–22. [Google Scholar]
- Kim, M.-J.; Pak, S.Y.; Kim, D.; Jung, S. Optimum Conditions for Extracting Ca from CKD to Store CO2 through Indirect Mineral Carbonation. KSCE J. Civ. Eng. 2017, 21, 629–635. [Google Scholar] [CrossRef]
- Irfan, M.F.; Hossain, S.M.Z.; Tariq, I.; Khan, N.A.; Tawfeeqi, A.; Goeva, A.; Wael, M. Modeling and Optimization of Aqueous Mineral Carbonation for Cement Kiln Dust Using Response Surface Methodology Integrated with Box-Behnken and Central Composite Design Approaches. Min. Metall. Explor. 2020, 37, 1367–1383. [Google Scholar] [CrossRef]
- Kunzler, C.; Alves, N.; Pereira, E.; Nienczewski, J.; Ligabue, R.; Einloft, S.; Dullius, J. CO2 Storage with Indirect Carbonation Using Industrial Waste. Energy Procedia 2011, 4, 1010–1017. [Google Scholar] [CrossRef]
- Available online: https://Federacciai.It/ (accessed on 26 May 2023).
- Federacciai. Rapporto di Sostenibilità; Federacciai: Milano, Italy, 2021. [Google Scholar]
- Mo, L.; Hao, Y.; Liu, Y.; Wang, F.; Deng, M. Preparation of Calcium Carbonate Binders via CO2 Activation of Magnesium Slag. Cem. Concr. Res. 2019, 121, 81–90. [Google Scholar] [CrossRef]
- Schultz, L.N.; Andersson, M.P.; Dalby, K.N.; Müter, D.; Okhrimenko, D.V.; Fordsmand, H.; Stipp, S.L.S. High Surface Area Calcite. J. Cryst. Growth 2013, 371, 34–38. [Google Scholar] [CrossRef]
- Parker, J.E.; Thompson, S.P.; Lennie, A.R.; Potter, J.; Tang, C.C. A Study of the Aragonite-Calcite Transformation Using Raman Spectroscopy, Synchrotron Powder Diffraction and Scanning Electron Microscopy. CrystEngComm 2010, 12, 1590. [Google Scholar] [CrossRef]
- Tao, M.-J.; Wang, Y.-J.; Li, J.-G.; Zeng, Y.-N.; Liu, S.-H.; Qin, S. Slurry-Phase Carbonation Reaction Characteristics of AOD Stainless Steel Slag. Processes 2021, 9, 2266. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, S.H.; Jeong, S.K.; Youn, M.H.; Nguyen, D.D.; Chang, S.W.; Kim, S.S. Calcium Extraction from Steelmaking Slag and Production of Precipitated Calcium Carbonate from Calcium Oxide for Carbon Dioxide Fixation. J. Ind. Eng. Chem. 2017, 53, 233–240. [Google Scholar] [CrossRef]
- Chang, E.-E.; Chen, C.-H.; Chen, Y.-H.; Pan, S.-Y.; Chiang, P.-C. Performance Evaluation for Carbonation of Steel-Making Slags in a Slurry Reactor. J. Hazard. Mater. 2011, 186, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Medas, D.; Cappai, G.; De Giudici, G.; Piredda, M.; Podda, S. Accelerated Carbonation by Cement Kiln Dust in Aqueous Slurries: Chemical and Mineralogical Investigation. Greenh. Gases Sci. Technol. 2017, 7, 692–705. [Google Scholar] [CrossRef]
- Alanyali, H.; Çöl, M.; Yilmaz, M.; Karagöz, Ş. Concrete Produced by Steel-Making Slag (Basic Oxygen Furnace) Addition in Portland Cement. Int. J. Appl. Ceram. Technol. 2009, 6, 736–748. [Google Scholar] [CrossRef]









| AOD_1 | LF_2 | EAF_1 | EAF_2 | EAF_3 + SiO2 | CKD | ||
|---|---|---|---|---|---|---|---|
| LOI | % | −0.7 | 5.7 | −3.3 | −3.0 | 1.1 | 29.9 |
| SiO2 | % | 23 ± 1 | 17.4 ± 0.6 | 10.9 ± 0.5 | 12.4 ± 0.5 | 27 ± 1 | 12.9 ± 0.5 |
| Al2O3 | % | 10.8 ± 0.5 | 10.4 ± 0.5 | 6.7 ± 0.4 | 7.9 ± 0.4 | 2.2 ± 0.2 | 2.5 ± 0.2 |
| Fe2O3 | % | 5.2 ± 0.4 | 12.8 ± 0.5 | 38 ± 1 | 44 ± 1 | 30 ± 1 | 0.2 ± 0.1 |
| CaO | % | 49 ± 1 | 36 ± 1 | 30 ± 1 | 23 ± 1 | 25 ± 1 | 45 ± 1 |
| MgO | % | 8.5 ± 0.4 | 11.6 ± 0.5 | 6.0 ± 0.4 | 6.2 ± 0.4 | 6.2 ± 0.4 | 0.5 ± 0.1 |
| SO3 | % | 0.2 ± 0.1 | 1.4 ± 0.2 | 0.3 ± 0.1 | 0.4 ± 0.1 | 0.2 ± 0.1 | 4.9 ± 0.4 |
| K2O | % | <LOQ | 0.5 ± 0.1 | <LOQ | <LOQ | 0.4 ± 0.1 | 1.0 ± 0.2 |
| Na2O | % | 0.8 ± 0.1 | 0.3 ± 0.1 | <LOQ | 0.1 ± 0.1 | 0.3 ± 0.1 | 2.7 ± 0.2 |
| TiO2 | % | 0.3 ± 0.1 | 1.2 ± 0.2 | 0.2 ± 0.1 | 0.6 ± 0.1 | 0.2 ± 0.1 | <LOQ |
| P2O5 | % | <LOQ | <LOQ | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 | <LOQ |
| Mn2O3 | % | 1.0 ± 0.1 | 1.6 ± 0.2 | 7.0 ± 0.4 | 7.0 ± 0.4 | 5.9 ± 0.4 | <LOQ |
| AOD_1 | LF_2 | EAF_1 | EAF_2 | EAF_2 + SiO2 | EAF_3 + SiO2 | CKD | |
|---|---|---|---|---|---|---|---|
| pHi | 13 | 13 | 12 | 10 | 12 | 13 | 12 |
| pi (bar) | 15.1 | 15.1 | 14.9 | 15.0 | 15.0 | 15.0 | 15.0 |
| pHf | 9 | 9 | 9 | 8 | 8.5 | 10 | 8 |
| pf (bar) | 2.2 | 4.0 | 6.0 | 7.2 | 7.1 | 5.5 | 7.6 |
| mi CO2 (g) | 2.53 | 2.59 | 2.44 | 2.59 | 2.69 | 2.69 | 2.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biava, G.; Zacco, A.; Zanoletti, A.; Sorrentino, G.P.; Capone, C.; Princigallo, A.; Depero, L.E.; Bontempi, E. Accelerated Direct Carbonation of Steel Slag and Cement Kiln Dust: An Industrial Symbiosis Strategy Applied in the Bergamo–Brescia Area. Materials 2023, 16, 4055. https://doi.org/10.3390/ma16114055
Biava G, Zacco A, Zanoletti A, Sorrentino GP, Capone C, Princigallo A, Depero LE, Bontempi E. Accelerated Direct Carbonation of Steel Slag and Cement Kiln Dust: An Industrial Symbiosis Strategy Applied in the Bergamo–Brescia Area. Materials. 2023; 16(11):4055. https://doi.org/10.3390/ma16114055
Chicago/Turabian StyleBiava, Giada, Annalisa Zacco, Alessandra Zanoletti, Giampiero Pasquale Sorrentino, Claudia Capone, Antonio Princigallo, Laura Eleonora Depero, and Elza Bontempi. 2023. "Accelerated Direct Carbonation of Steel Slag and Cement Kiln Dust: An Industrial Symbiosis Strategy Applied in the Bergamo–Brescia Area" Materials 16, no. 11: 4055. https://doi.org/10.3390/ma16114055