1. Introduction
Bone is a natural organic/inorganic composite material that exhibits a complex hierarchical structure, ranging from arrangements in the nanometer range to structures on a macroscopic level. At the lowest level, polypeptide chains are wound together to form triple helices, which in turn are assembled into fibrils. The collagen fibrils act as templates for mineralization, in which the apatite crystals grow on the collagen fibrils. These mineralized collagen fibrils assemble into fibril bundles, which in turn form osteons. The osteons represent the basic structural unit of the cortex, also compact bone, and consist of concentric bone lamellae arranged around the central Haversian canal. The Haversian canals run through blood vessels and nerve cords. The cancellous bone has a spongy network of trabeculae, which consist of lamellar bone [
1,
2]. This highly porous trabeculae network with approximately 50–90% porosity [
3] is filled with bone marrow, in which also hematopoietic and mesenchymal stem cells find their origin [
4,
5]. The complex hierarchical structure of bone is a reason for its special mechanical stability combining elasticity and strength [
6]. The mechanical properties of bone originate from its structure. The outer compact bone exhibits elastic modulus of 3–30 GPa and the inner cancellous bone has elastic modulus of 0.02–2 GPa. In addition, the construction of cancellous bone enables the exchange of substances and the supply of the bone cells [
7]. Bone tissue is subject to a constant remodeling process in which bone is continuously replaced by osteoclasts and is rebuilt by osteoblasts.
Silica/collagen composites produced by sol–gel process are biomimetic materials of organic and inorganic components, equipped with a structure over several structural levels [
8]. So far, the focus has been on monolithic silica/collagen Xerogels, which have been investigated for their suitability as bone substitute materials [
8,
9,
10,
11,
12]. Owing to their hybrid character and their structure over several structural levels, silica/collagen Xerogels have mechanical strengths in areas relevant to the bone [
9]. Moreover, silica/collagen Xerogels have been shown to be bioactive, degradable, and resorbable, as well as to affect proliferation and osteogenic differentiation of human bone marrow stromal cells and osteoclastogenesis [
9,
10,
11,
12]. With all these properties, silica/collagen Xerogels match a lot of requirements to an ideal bone implant. However, macroporosity with pore sizes about 200–350 µm is another critical and desirable parameter for the success of bone implant. These structure sizes and resulting void spaces are mainly relevant for cell ingrowth and their nutrition especially in large biomaterial constructs. So far, this was lacking in case of monolithic silica/collagen Xerogels.
The fabrication of macroporous implants can be associated with scaffolds, which are the key elements of tissue engineering. For such tissue-engineered bone implants, various manufacturing methods have been applied in the last decades. However, methods like freeze drying, electrospinning, salt leaching, gas foaming or fiber deposition are not suitable to fabricate accurate scaffolds with a designed macroporosity and with defined pore sizes [
13]. Moreover, properties related to the individual adjustment to diseases and/or patients gain importance [
14]. To match patient specific shapes of fractures or lesions, as well as material macroporosity, interconnected pores, and defined pore sizes, additive manufacturing, especially 3D printing, is a widely used method [
15].
By the help of additive manufacturing, material is added in a layer-by-layer manner to create a 3D implant [
16]. Three-dimensional printing, a type of additive manufacturing, has the focus on printing by the help of inks. In this way, scaffolds can be fabricated by controlling shape, porosity, and pore size, as well as the interconnectivity of pores. One key element of 3D printing is the ink used. Therefore, a wide range of biomaterial inks were developed [
17]. Biomaterial inks to create bone scaffolds combine both requirements on bone implant materials at all like excellent performance in mechanical stability, bio- and cytocompatibility, and degradation and resorption, as well as requirements on their processing. Requirements on processing are dependent on the type of 3D-printing technique.
To process biomaterial inks and to fabricate bone implants by 3D printing, currently, there are three main techniques: material jetting, material extrusion, and vat polymerization. Material jetting is a computer controlled technique based on the precise deposition of biomaterial ink droplets of microliter volumes onto a substrate [
18]. Material jetting is widely applied in tissue engineering, using mostly hydrogel inks, like gelatin or alginate. Material extrusion is another computer controlled 3D-printing technique. Biomaterial inks for extrusion-based 3D printing are usually pastes, solutions, or dispersions that can be extruded by the coordinated motion of pneumatic pressure or plunger- or screw-based pressure through a nozzle or a needle onto a substrate [
19]. The extruded biomaterial ink forms a continuous filament that is applied layer-by-layer to build 3D patterns that in turn form the scaffold in the desired shape. A fine resolution can be reached by the usage of related microscale nozzles or needles. The third technique—vat polymerization—is based on a liquid photopolymer resin stored in a vat. This liquid polymer is selectively polymerized at the surface of the vat. Polymerization is induced by a low-power ultraviolet (UV) light source [
20]. A new thin layer is spread over the solid surface, while the
z-axis is moving down. This process is repeated to fabricate a complete scaffold. The resolution of this technique varies from pico- to micro-scale, mostly depending on the used material [
21].
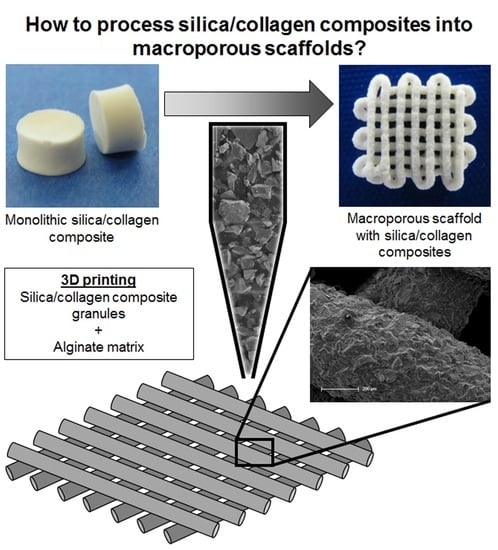
In the present study, the advantage of excellent material properties of multiphasic silica/collagen Xerogels is to be combined with the advantages of extrusion-based 3D plotting. Thus, it is a successful first-time proof of principle to process granules made of silica/collagen composites in a viscoelastic matrix by 3D plotting.
2. Materials and Methods
2.1. Preparation of Xerogel/Alg Paste
First, Xerogel monoliths were prepared as described previously and shown in
Figure 1. In brief, bovine tropocollagen type I (GfN Herstellung von Naturextrakten GmbH, Wald-Michelbach, Germany) was dialyzed (MWCO 12–14 kDa, Roth, Karlsruhe, Germany) against deionized water for 7 days, fibrillized (30 mM neutral sodium phosphate buffer solution), and then lyophilized (until frozen liquid was removed; Christ Alpha1-4 laboratory freeze-dryer, Martin Christ Gefriertrocknungsanlagen GmbH, Osterode am Harz, Germany). Collagen was resuspended in 0.05 M Tris (pH 8, Roth, Karlsruhe, Germany) to a homogenous suspension (30 mg/mL). Silicic acid was prepared by hydrolysis of tetraethoxysilane (TEOS, 99%, Sigma-Aldrich, St. Luis, MO, USA; molar ratio TEOS/water = 1/4) under acidic conditions (0.01 M HCl). TEOS colloids were increasingly crushed by vigorous stirring (magnetic stirrer, 700 rpm), leading to a cloudy solution. After approximately 20 min, hydrolysis took place. Owing to the polymerization processes, there is always a mixture of silicic acids with different degrees of polymerization. To decrease polymerization processes, the solution was immediately cooled on ice for 30 min and then used for Xerogel production within one hour. For hydrogels with biphasic composition Xerogel, 30 wt.% bovine collagen and 70 wt.% silica were vigorously stirred, using a vortex mixer. After stabilization for 3 days, a climate chamber (Espec SH-221, Kita-ku, Japan) at 37 °C and 95% relative humidity, followed by a climate ramp, to achieve ambient conditions, was used to dry hydrogels until mass constancy. Afterwards, Xerogel granules were produced by grinding Xerogel monoliths, using a mixer mill (MM 400, Retsch, Haan, Germany). The resulting Xerogel granules were classified by means of a vibratory sieve shaker (AS 200, Retsch, Haan, Germany) and mesh sieves (25, 40, 71, and 125 μm).
Alginate (alginic acid sodium salt from brown algae, Sigma-Aldrich, St. Luis, MO, USA) was dissolved in phosphate buffered saline (PBS, Biochrom GmbH, Berlin, Germany), under vigorous stirring, to obtain a homogenous solution as matrix phase for the biomaterial ink. For degassing, alginate was centrifuged (900 rcf, 2 min). For biomaterial ink preparation, Xerogel granules were added to alginate in various mass ratios of Xerogel granules to alginate (Xerogel/Alg ratio) and distributed homogeneously, hereafter referred to as Xerogel/Alg paste. Xerogel/Alg paste was transferred to cartridges and centrifuged (1200 rcf, 3 min).
2.2. Rheological Characterization of Xerogel/Alg Pastes
A plate rheometer (Haake Mars II, Thermo Fisher Scientific, Waltham, MA, USA) with plate cone diameter of 20 mm and plate-plate-distance of 1 mm was used for rheological characterization of Xerogel/Alg pastes. Viscosity of pastes was measured in rotation mode with shear rates in ranges of 0.01–40 s−1. Amplitude sweep was used to evaluate viscoelastic region. Therefore, storage modulus as well as loss modulus were measured in oscillation mode for shear stress in ranges of 0.1–25,000 Pa working with constant oscillatory frequency of 1 s−1.
2.3. Scaffold Fabrication
For extrusion, Xerogel/Alg pastes were filled in 10 mL cartridges with conical needles with 410–840 µm in inner diameter (Globaco GmbH, Rödermark, Germany). Extrusion was performed via compressed air (70–540 kPa air pressure). Scaffolds (6 × 6 mm
2) with alternating layer pattern (0°/90°, ABAB) were fabricated with a 3DDiscovery (RegenHU, Villaz-St-Pierre, Swiss), working with 0.5–4 mm/s print head speed. Plotting parameters are summarized in
Table 1. After plotting, scaffolds were immersed in CaCl
2 solution (1 M) for 5 min, to allow crosslinking, and dried at 37 °C (Xerogel/Alg scaffold). To estimate the dependency of the thickness of a plotted strand and the nozzle size, photographs of as-printed scaffolds, as well as of SEM images of dried scaffolds, were analyzed, using ImageJ (Vers. 1.53e, W. Rasband, National Institutes of Health, Bethesda, MD, USA). Strand diameter was measured at least 6 times each in horizontal and vertical orientation for calculating mean and standard deviation as a function of printing parameters.
2.4. Scanning Electron Microscopy
The 3D-plotted Xerogel/Alg scaffolds were characterized for surface morphology, as well as using a cross-section obtained by cutting with a diamond wire saw. Samples carbon coated on aluminum stubs were studied by using an ESEM XL 30 (Thermo Fisher Scientific, Waltham, MA, USA) scanning electron microscope (SEM). SEM investigations were carried out under high vacuum, an acceleration voltage of 3 kV, and detecting secondary electrons.
4. Discussion
The aim of the present study was to evaluate 3D plotting parameters to process silica/collagen composites. These silica/collagen composites were shown earlier to be bioactive, degradable, and resorbable, and they exhibit cell-regulating properties, such as affecting proliferation and osteogenic differentiation of human bone marrow stromal cells and osteoclastogenesis [
8,
9,
10,
11,
12]. For the success of a bone implant, macroporosity and pore sizes in bone-relevant ranges are critical parameters that were, so far, lacking in the case of monolithic silica/collagen Xerogels. Macroporosity, interconnectivity of pores, and pore sizes can be controlled easily and precisely by additive manufacturing. Moreover, the fabrication of implants with patient-specific shapes of fractures or lesions is possible by using extrusion-based 3D printing. Thus, the advantage of excellent material properties of multiphasic silica/collagen Xerogels was to be combined with the advantages of 3D printing.
Out of the three main techniques of 3D bioprinting—material jetting, material extrusion, and vat polymerization—extrusion-based 3D printing, also 3D plotting, was chosen because of the planned composition of the scaffold. The aim was to have the silica/collagen Xerogel as the effective main component that gives the cell-regulating properties to the later macroporous scaffold. Material jetting, in combination with silica/collagen Xerogels, was not suitable. Xerogels should have a representative size, to give, especially, their cell-regulating properties to the scaffold. Vat polymerization could be suitable, using a photo-curable polymer matrix. Until now, vat polymerization for bone tissue engineering is limited in terms of the amounts of additives, e.g., ceramic powder [
20]. By using extrusion-based 3D-printing, Xerogel granules can be used as the filler material incorporated in a matrix suitable for plotting.
Granular silica/collagen Xerogels were incorporated in a high amount, as an effective main component embedded in a viscoelastic matrix. Among the high amount of granules, irregular and ridged shapes of these granules (
Figure 4 and
Figure 5) are a challenge for the extrusion based plotting process. Owing to its viscoelastic properties, alginate shows good handling for 3D plotting. By mixing Xerogel granules as disperse phase with an alginate matrix as continuous phase in various mass ratios, as well as with different concentrations of the alginate, Xerogel/Alg pastes (biomaterial inks) were prepared. The present study demonstrates that these Xerogel/Alg pastes were suitable for extrusion through conical needles, using a 3D plotter.
The main task for implementation of the 3D-plotting process was the optimization of the mechanical properties of the biomaterial to be plotted. Therefore, the biomaterial ink has to fulfill two requirements. First, the biomaterial ink should exhibit shear-thinning flow behavior to enable 3D plotting under low pressure. Second, biomaterial ink should be elastic enough for the fabrication of stable scaffold strands, as well as for dimensional stability of scaffold geometry after extrusion [
23,
24]. Shear-thinning behavior was observed for all pastes based on both 13 and 17 wt.% alginate (
Figure 2a,b). Hence, all tested Xerogel/Alg pastes are suitable for the processing under low pressure. The evidence of this was provided with the execution of the process using a 3D plotter.
In order to evaluate viscoelasticity of the pastes and to predict dimensional stability of the extruded scaffold strands after 3D plotting, storage modulus G’, and loss modulus G’’ of the shear modulus were measured. The storage modulus G’ indicates elastic deformability, and the loss modulus G’’ indicates viscous deformability to the viscoelasticity of the paste. Thus, a comparison of both shear moduli at low shear stress provides information on the dimensional stability. For G’ > G’’, elastic contribution of viscoelasticity dominated and extruded strands will therefore be dimensionally stable. That was predicted by amplitude sweep tests (
Figure 2c,d) for all Xerogel/Alg pastes made of 17 wt.% alginate (component ratios 1:3.33 to 1:2), as well as for Xerogel/Alg pastes made of 13 wt.% alginate, with component ratios of 1:2.5 and 1:2. SEM images of scaffolds fabricated with Xerogel/Alg pastes of 17 wt.% alginate show that strands collapsed after extrusion (
Figure 4). Most probably, this is a result of gravity. In contrast to observations in the present study, it has been reported that pure alginate at concentrations of 16.7 wt.% in PBS was processed successfully by 3D plotting [
22].
For the latter, 13 wt.% alginate with component ratios of 1:2.5 and 1:2, this could be proved by generated 3D scaffolds with excellent dimensional stability (
Figure 3 and
Figure 5). In the opposite case, when G’’ > G’, viscous contribution of viscoelasticity dominates and extruded strands will not be stable as present for pure 13 wt.% alginate and pure 17 wt.% alginate. Beside macroscopic observation, SEM images (
Figure 5) confirm the dimensional stability of Xerogel/Alg scaffolds.
In general, viscous contribution of viscoelasticity dominates for pastes of pure alginate (13 and 17 wt.%). When comparing pastes of different alginate concentrations, it has to be mentioned that the increase in alginate concentration from 13 to 17 wt.% led to an increase in shear modulus at all, as well as to approximately similar values of G’ and G’’ in the idle state. It can be assumed that the increased density of polymer chains led to a higher degree of formed inter- and intramolecular hydrogen bonds between homopolymeric chain segments. Moreover, rotation of blocks of α-L-guluronic acid is restricted. Both may cause a higher chain immobility and chain stiffness at the molecular level, which macroscopically leads to an increase in both modules with a higher increase in the storage module G’ in the linear viscoelastic region [
25].
For all Xerogel-containing pastes, except 13 wt.% alginate with component ratio of 1:3.33, G’ was higher than G’’ (G’ > G’’). Hence, the elastic contribution of viscoelasticity dominated in the Xerogel-containing pastes. Possibly, the irregular and ridged shape of the Xerogel granules causes physical interactions between the Xerogel and the alginate matrix contributing to the dimensional stability after plotting. Strongest characteristic of viscoelastic behavior was observed for 17 wt.% alginate with component ratio of 1:2. For that Xerogel/Alg paste, G’ was much higher than G’’, leading to higher shear stress that was necessary to extrude this paste through conical dispensing needles of size G22 (inner diameter of 410 µm).
After that successful proof of plottability further, investigation on biocompatibility, degradation and mechanical properties will be performed in the future. The properties of the Xerogel [
8,
9,
10,
12] as the main component will mainly influence biocompatibility, degradation and mechanical properties of the 3D-plotted Xerogel/Alg scaffolds. Moreover, the plotting process will be investigated in-depth by optical coherence tomography.
The present study is a proof of principle that demonstrates the suitable processing of silica/collagen Xerogel granules via 3D plotting. These composite granules are hybrid materials consisting of several microstructural levels [
8]. As shown by other studies, 3D plotting of less complex materials, such as hydroxyapatite or bioglass particles [
26,
27,
28], can be used to generate additional macrostructural levels. In the present case, 3D-plotting of silica/collagen hybrids generates two further levels: (1) hybrids embedded in the alginate matrix (called double-hybrid) and (2) double-hybrids processed to 3D scaffolds consisting of a tailorable solid phase and a continuous pore phase.