Effect of Grain Size on the Corrosion Behavior of Fe-3wt.%Si-1wt.%Al Electrical Steels in Pure Water Saturated with CO2
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Materials
2.2. Metallographic Analysis
2.3. Gravimetric Measurement
2.4. Electrochemical Measurement
2.5. Surface Analysis
3. Results and Discussion
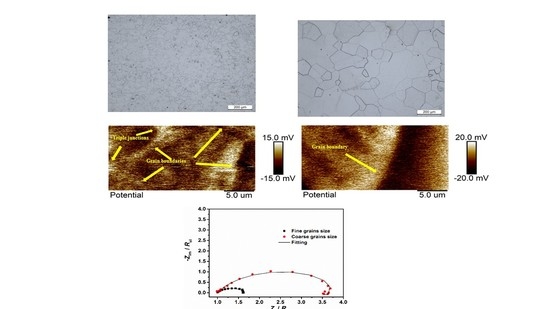
3.1. Metallographic Measurement
3.2. Gravimetric Measurements
3.3. Electrochemical Measurements
3.4. Morphological Analysis
4. Conclusions
- The gravimetric results indicated that the grain refinement decreases the corrosion resistance of the steel.
- The EIS measurements showed that the coarse-grained sample displayed a higher capacitive loop compared to fine-grained steel. This result is related to the formation of a thicker and more stable protective corrosion product layer.
- The potentiodynamic measurements showed that the corrosion current density of the coarse-grained steel was much smaller compared fine-grained steel. Both the anodic and cathodic current densities were found to be lower for the coarse-grained steel.
- The SEM-EDS and XPS analyses confirmed presence of a thicker and more homogenous protective layer on the coarse-grained steel, consisting mainly of Al2O3, SiO2, and traces of FeCO3.
- The Volta potential measurements showed potential differences between the grains and grain boundaries, indicating a higher electrochemical activity in these regions, which would couse a preferential dissolution of grains.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, Y.; Jiao, H.; Qiu, W.; Misra, R.D.K.; Li, J. Effect of cold rolling process on microstructure, texture and properties of strip cast Fe-2.6% Si Steel. Materials 2018, 11, 1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, C.; Wang, H.; Wu, Y.; Wang, H. Joining of the laminated electrical steels in motor manufacturing: A Review. Materials 2020, 13, 4583. [Google Scholar] [CrossRef] [PubMed]
- Petryshynets, I.; Kováč, F.; Petrov, B.; Falat, L.; Puchý, V. Improving the magnetic properties of non-oriented electrical steels by secondary recrystallization using dynamic heating conditions. Materials 2019, 12, 1914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.; Park, S.; Huh, M.; Kim, J.; Engler, O. Effect of texture and grain size on magnetic flux density and core loss in non-oriented electrical steel containing 3.15% Si. J. Magn. Magn. Mater. 2014, 354, 324–332. [Google Scholar] [CrossRef]
- Palumbo, G.; Górny, M.; Banaś, J. Corrosion inhibition of pipeline carbon steel (N80) in CO2-saturated chloride (0.5 M of KCl) solution using gum arabic as a possible environmentally friendly corrosion inhibitor for shale gas industry. J. Mater. Eng. Perform. 2019, 28, 6458–6470. [Google Scholar] [CrossRef] [Green Version]
- Palumbo, G.; Kollbek, K.; Wirecka, R.; Bernasik, A.; Górny, M. Effect of CO2 partial pressure on the corrosion inhibition of N80 carbon steel by gum arabic in a CO2-water saline environment for shale oil and gas industry. Materials 2020, 13, 4245. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Ma, L.; Cheng, X.; Li, X. Effect of grain size and crystallographic orientation on the corrosion behaviors of low alloy steel. J. Alloys Compd. 2021, 857, 158258. [Google Scholar] [CrossRef]
- Onyeji, L.; Kale, G. Preliminary investigation of the corrosion behavior of proprietary micro-alloyed steels in aerated and deaerated brine solutions. J. Mater. Eng. Perform. 2017, 26, 5741–5752. [Google Scholar] [CrossRef] [Green Version]
- Di Schino, A.; Barteri, M.; Kenny, J.M. Grain size dependence of mechanical, corrosion and tribological properties of high nitrogen stainless steels. J. Mater. Sci. 2003, 38, 3257–3262. [Google Scholar] [CrossRef]
- Di Schino, A.; Kenny, J.M. Effects of the grain size on the corrosion behavior of refined AISI 304 austenitic stainless steels. J. Mater. Sci. Lett. 2002, 21, 1631–1634. [Google Scholar] [CrossRef]
- Ralston, K.D.; Birbilis, N. Effect of grain size on corrosion: A review. Corrosion 2010, 66, 075005. [Google Scholar] [CrossRef]
- Li, Y.; Wang, F.; Liu, G. Grain size effect on the electrochemical corrosion behavior of surface nanocrystallized low-carbon steel. Corrosion 2004, 60, 891–896. [Google Scholar] [CrossRef]
- Palumbo, G.; Thorpe, S.; Aust, K. On the contribution of triple junctions to the structure and properties of nanocrystalline materials. Scr. Metall. Mater. 1990, 24, 1347–1350. [Google Scholar] [CrossRef]
- Soleimani, M.; Mirzadeh, H.; Dehghanian, C. Effect of grain size on the corrosion resistance of low carbon steel. Mater. Res. Express 2019, 7, 016522. [Google Scholar] [CrossRef]
- Chen, Y.T.; Zhang, K.G. Influence of grain size on corrosion resistance of a HSLA steel. Adv. Mater. Res. 2012, 557–559, 143–146. [Google Scholar] [CrossRef]
- Zeiger, W.; Schneider, M.; Scharnweber, D.; Worch, H. Corrosion behaviour of a nanocrystalline FeA18 alloy. Nanostructured Mater. 1995, 6, 1013–1016. [Google Scholar] [CrossRef]
- Wang, S.G.; Shen, C.B.; Long, K.; Zhang, T.; Wang, F.H.; Zhang, Z.D. The electrochemical corrosion of bulk nanocrystalline ingot iron in acidic sulfate solution. J. Phys. Chem. B 2006, 110, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Seikh, A.H. Influence of heat treatment on the corrosion of microalloyed steel in sodium chloride solution. J. Chem. 2013, 2013, 587514. [Google Scholar] [CrossRef] [Green Version]
- ASTM International. ASTM-E112. Standard Test Methods for Determining Average Grain Size; ASTM-E112: West Conshohocken, PA, USA, 2013. [Google Scholar]
- ASTM International. ASTM-G102. Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements; ASTM-G102: West Conshohocken, PA, USA, 1994. [Google Scholar]
- Palumbo, G.; Berent, K.; Proniewicz, E.; Banaś, J. Guar gum as an eco-friendly corrosion inhibitor for pure aluminium in 1-M HCl solution. Materials 2019, 12, 2620. [Google Scholar] [CrossRef] [Green Version]
- Mustafa, A.H.; Ari-Wahjoedi, B.; Ismail, M.C. Inhibition of CO2 corrosion of X52 steel by imidazoline-based inhibitor in high pressure CO2-water environment. J. Mater. Eng. Perform. 2012, 22, 1748–1755. [Google Scholar] [CrossRef]
- Islam, M.A.; Farhat, Z.N. Characterization of the corrosion layer on pipeline steel in sweet environment. J. Mater. Eng. Perform. 2015, 24, 3142–3158. [Google Scholar] [CrossRef]
- Zhang, G.; Cheng, Y. Electrochemical characterization and computational fluid dynamics simulation of flow-accelerated corrosion of X65 steel in a CO2-saturated oilfield formation water. Corros. Sci. 2010, 52, 2716–2724. [Google Scholar] [CrossRef]
- Dugstad, A. Fundamental aspects of CO2 metal loss corrosion—Part 1: Mechanism. In Corrosion 2006; NACE International: San Diego, CA, USA, 2006; p. 18. [Google Scholar]
- Heuer, J.; Stubbins, J. An XPS characterization of FeCO3 films from CO2 corrosion. Corros. Sci. 1999, 41, 1231–1243. [Google Scholar] [CrossRef]
- Barrera, A.; Tzompantzi, F.; Campa-Molina, J.; Casillas, J.E.; Pérez-Hernández, R.; Ulloa-Godinez, S.; Velásquez, C.; Arenas-Alatorre, J. Photocatalytic activity of Ag/Al2O3-Gd2O3 photocatalysts prepared by the sol–gel method in the degradation of 4-chlorophenol. RSC Adv. 2018, 8, 3108–3119. [Google Scholar] [CrossRef] [Green Version]
- Dong, B.; Zeng, D.; Yu, Z.; Cai, L.; Shi, S.; Yu, H.; Zhao, H.; Tian, G. Corrosion mechanism and applicability assessment of N80 and 9Cr steels in CO2 auxiliary steam drive. J. Mater. Eng. Perform. 2019, 28, 1030–1039. [Google Scholar] [CrossRef]
- Garcia, S.; Rosenbauer, R.; Palandri, J.; Maroto-Valer, M. Sequestration of non-pure carbon dioxide streams in iron oxyhydroxide-containing saline repositories. Int. J. Greenh. Gas Control. 2012, 7, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Ansari, K.R.; Quraishi, M.A.; Lgaz, H. Effect of electron donating functional groups on corrosion inhibition of J55 steel in a sweet corrosive environment: Experimental, density functional theory, and molecular dynamic simulation. Materials 2018, 12, 17. [Google Scholar] [CrossRef] [Green Version]
- Örnek, C.; Engelberg, D. SKPFM measured Volta potential correlated with strain localisation in microstructure to understand corrosion susceptibility of cold-rolled grade 2205 duplex stainless steel. Corros. Sci. 2015, 99, 164–171. [Google Scholar] [CrossRef]













| Element | Sample | |
|---|---|---|
| wt.% | 200 | 300 |
| C | 0.011 ± 0.01 | 0.007 ± 0.01 |
| Si | 2.93 ± 0.17 | 3.24 ± 0.11 |
| Mn | 0.22 ± 0.03 | 0.18 ± 0.02 |
| P | 0.03 ± 0.01 | 0.026 ± 0.03 |
| S | 0.004 ± 0.001 | 0.003 ± 0.001 |
| Al | 0.91 ± 0.13 | 1.05 ± 0.08 |
| Fe | Bal. | Bal. |
| 200 | 300 | ||||
|---|---|---|---|---|---|
| Average grain size number (G) | Average diameter (µm) | Average grain area (µm2) | Average grain size number (G) | Average diameter (µm) | Average grain area (µm2) |
| 10.1 ± 0.23 | 10.8 ± 0.87 | 115.6 ± 20.9 | 4.9 ± 0.32 | 65.3 ± 7.49 | 4265.9 ± 499.7 |
| Sample | Weight Loss (mg) | Corrosion Rate (mm y−1) |
|---|---|---|
| 200 | 3.57 ± 0.25 | 0.25 ± 0.02 |
| 300 | 0.79 ± 0.04 | 0.12 ± 0.01 |
| Sample | Time (h) | Rs (Ω cm2) | Q1 (mΩ−1 sn cm−2) | n | Rct (Ω cm2) | L (H cm2) | RL (Ω cm2) | χ2 (10−4) |
|---|---|---|---|---|---|---|---|---|
| 200 | 3 | 0.96 | 0.48 ± 0.06 | 0.57 ± 0.02 | 0.47 ± 0.03 | 0.27 ± 0.03 | 0.14 ± 0.011 | 1.05 |
| 6 | 0.98 | 0.38 ± 0.03 | 0.59 ± 0.04 | 0.45 ± 0.05 | 0.15 ± 0.02 | 0.01 ± 0.005 | 0.99 | |
| 12 | 0.98 | 0.36 ± 0.03 | 0.58 ± 0.05 | 0.54 ± 0.06 | 0.19 ± 0.04 | 0.01 ± 0.003 | 0.38 | |
| 18 | 0.97 | 0.26 ± 0.02 | 0.63 ± 0.03 | 0.65 ± 0.03 | 0.12 ± 0.02 | 0.04 ± 0.001 | 0.46 | |
| 24 | 0.99 | 0.19 ± 0.01 | 0.67 ± 0.05 | 0.64 ± 0.03 | 0.07 ± 0.01 | 0.07 ± 0.002 | 1.6 | |
| 300 | 3 | 0.95 | 0.04 ± 0.001 | 0.61 ± 0.04 | 2.32 ± 0.16 | 0.24 ± 0.01 | 0.70 ± 0.06 | 2.02 |
| 6 | 0.98 | 0.04 ± 0.001 | 0.65 ± 0.03 | 2.35 ± 0.21 | 0.25 ± 0.02 | 0.69 ± 0.02 | 2.06 | |
| 12 | 0.89 | 0.04 ± 0.003 | 0.69 ± 0.04 | 2.03 ± 0.22 | 0.64 ± 0.01 | 0.49 ± 0.02 | 1.99 | |
| 18 | 0.98 | 0.05 ± 0.005 | 0.71 ± 0.03 | 2.26 ± 0.15 | 0.36 ± 0.02 | 0.52 ± 0.03 | 1.41 | |
| 24 | 0.97 | 0.05 ± 0.003 | 0.72 ± 0.02 | 2.61 ± 0.28 | 0.36 ± 0.01 | 0.51 ± 0.05 | 0.94 |
| Sample | Ecorr (V vs. SCE) | −βc (V dec−1) | icorr (μA cm−2) | CR (mm y−1) |
|---|---|---|---|---|
| 200 | −0.696 ± 0.015 | 0.433 ± 0.087 | 24.44 ± 1.87 | 0.28 ± 0.02 |
| 300 | −0.680 ± 0.011 | 0.588 ± 0.051 | 12.98 ± 2.57 | 0.15 ± 0.03 |
| Sample | Time (h) | Rs (Ω cm2) | Q1 (mΩ−1 sn cm−2) | n | Rct (Ω cm2) | L (H cm2) | RL (Ω cm2) | χ2 (10−4) |
|---|---|---|---|---|---|---|---|---|
| 200 | 24 | 10.04 | 0.46 ± 0.03 | 0.78 ± 0.03 | 421.10 ± 28.53 | 50.11 ± 9.07 | 49.22 ± 8.59 | 8.27 |
| 300 | 24 | 10.26 | 0.30 ± 0.06 | 0.80 ± 0.05 | 968.30 ± 40.89 | 60.10 ± 10.03 | 31.81 ± 6.89 | 5.69 |
| Sample | Ecorr (V vs. SCE) | −βc (V dec−1) | icorr (μA cm−2) | CR (mm y−1) |
|---|---|---|---|---|
| 200 | −0.735 ± 0.019 | 0.517 ± 0.044 | 70.42 ± 2.34 | 0.82 ± 0.03 |
| 300 | −0.746 ± 0.016 | 0.471 ± 0.067 | 29.43 ± 1.37 | 0.34 ± 0.02 |
| Element (wt.%) | 200 | 300 | ||
|---|---|---|---|---|
| 1 | 2 | 1 | 2 | |
| C | 11.97 ± 2.95 | 23.04 ± 2.17 | 21.78 ± 0.08 | 20.83 ± 1.35 |
| O | 7.11 ± 1.62 | 14.75 ± 1.04 | 10.71 ± 1.44 | 14.38 ± 1.03 |
| Al | 0.90 ± 0.08 | 3.54 ± 0.53 | 4.96 ± 0.55 | 7.10 ± 0.47 |
| Si | 2.60 ± 0.14 | 3.39 ± 0.30 | 3.19 ± 0.10 | 3.50 ± 0.05 |
| P | 0.09 ± 0.01 | 0.24 ± 0.02 | 0.10 ± 0.01 | 0.11 ± 0.01 |
| S | 0.08 ± 0.03 | 0.11 ± 0.02 | 0.11 ± 0.01 | 0.06 ± 0.01 |
| Mn | 0.13 ± 0.03 | 0.06 ± 0.01 | 0.07 ± 0.01 | 0.13 ± 0.01 |
| Fe | 77.12 ± 4.58 | 54.87 ± 1.42 | 59.12 ± 2.37 | 50.88 ± 5.55 |
| Peak Assignment | 200 | 300 | ||
|---|---|---|---|---|
| Binding Energy (eV) | %Area | Binding Energy (eV) | %Area | |
| C1s | - | 23.0 | - | 14.5 |
| C–C, C–H | 284.8 | 47.5 | 284.8 | 52.4 |
| C–O, C–OH | 286.5 | 35.2 | 286.3 | 24.5 |
| C=O | 288.7 | 17.3 | 288.5 | 14.2 |
| - | - | 289.8 | 8.9 | |
| Al2p | - | 12.0 | - | 14.5 |
| Al2O3 | 74.7 | 85.8 | 74.7 | 72.6 |
| AlOOH | 77.5 | 14.2 | 77.3 | 27.4 |
| Si2p | - | 4.6 | - | 7.2 |
| SiOx | 101.8 | 66.7 | 102.1 | 66.7 |
| splitting | 102.5 | 33.3 | 102.7 | 33.3 |
| O1s | - | 53.7 | - | 54.0 |
| O–Me | 530 | 36.6 | 530.1 | 38.2 |
| O–C | 531.6 | 45.3 | 531.6 | 38.9 |
| O=C | 532.9 | 18.1 | 533.0 | 17.8 |
| - | - | 534.6 | 5.1 | |
| Fe2p | - | 5.7 | - | 8.3 |
| Fe3+ | 710.5 | 44.5 | 710.7 | 51.0 |
| Fe3+ | 713.2 | 15.2 | 714.0 | 15.3 |
| satellite | 717.6 | 13.2 | 718.6 | 9.7 |
| - | 723.9 | 22.2 | 724.0 | 19.1 |
| - | 726.7 | 4.9 | 726.5 | 4.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palumbo, G.; Dunikowski, D.; Wirecka, R.; Mazur, T.; Lelek-Borkowska, U.; Wawer, K.; Banaś, J. Effect of Grain Size on the Corrosion Behavior of Fe-3wt.%Si-1wt.%Al Electrical Steels in Pure Water Saturated with CO2. Materials 2021, 14, 5084. https://doi.org/10.3390/ma14175084
Palumbo G, Dunikowski D, Wirecka R, Mazur T, Lelek-Borkowska U, Wawer K, Banaś J. Effect of Grain Size on the Corrosion Behavior of Fe-3wt.%Si-1wt.%Al Electrical Steels in Pure Water Saturated with CO2. Materials. 2021; 14(17):5084. https://doi.org/10.3390/ma14175084
Chicago/Turabian StylePalumbo, Gaetano, Dawid Dunikowski, Roma Wirecka, Tomasz Mazur, Urszula Lelek-Borkowska, Kinga Wawer, and Jacek Banaś. 2021. "Effect of Grain Size on the Corrosion Behavior of Fe-3wt.%Si-1wt.%Al Electrical Steels in Pure Water Saturated with CO2" Materials 14, no. 17: 5084. https://doi.org/10.3390/ma14175084