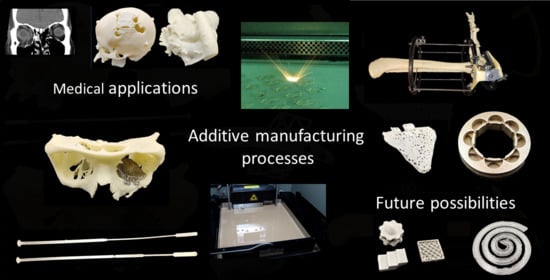
Additive Manufacturing Processes in Medical Applications
Abstract
:1. Introduction
- What are the basic benefits of AM in medical applications?
- What AM processes based on ISO/ASTM process classification are utilized in medical applications?
- What are the example materials utilized in founded process and application combinations?
- Based on the findings, what are the process and application areas that could show future scientific potential?
2. Additive Manufacturing Processes
3. Medical Applications of Additive Manufacturing
- Medical models;
- Implants;
- Tools, instruments and parts for medical devices;
- Medical aids, supportive guides, splints and prostheses;
- Biomanufacturing.
3.1. Medical Models
3.2. Implants
3.3. Tools, Instruments and Parts for Medical Devices
3.4. Medical Aids, Supportive Guides, Splints and Prostheses
3.5. Biomanufacturing
4. Different AM Processes in Medical Applications
5. Discussion and Conclusions
5.1. Limitations of Previous Reviews
5.2. Processes Utilized Rarely—DED, SL
5.3. Well Established Processes—PBF, MEX, VP
5.4. Processes Well Established in Some Application Areas—MJ, BJ
5.5. Future Possibilities
- Directed energy deposition—repairing medical parts especially in tools, instruments and parts for medical devices;
- Sheet lamination—multi-metal parts in medicine, especially in tools, instruments and parts for medical devices;
- Material extrusion—composite parts and multi-material, especially in medical aids, supportive guides, splints and prostheses;
- Material extrusion—metal parts especially in implants and tools, instruments and parts for medical devices;
- Binder jetting—metal parts especially in implants and tools, instruments and parts for medical devices;
- Material jetting—multi-material parts, especially in medical models and biomanufacturing.
Funding
Conflicts of Interest
References
- ASTM International. ISO/ASTM52900—15 Standard Terminology for Additive Manufacturing—General Principles—Terminology; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Lehtinen, P.; Väisänen, T.; Salmi, M. The effect of local heating by laser irradiation for aluminum, deep drawing steel and copper sheets in incremental sheet forming. Phys. Procedia 2015, 78, 312–319. [Google Scholar] [CrossRef]
- Casalino, G.; Ludovico, A.D.; Ancona, A.; Lugarà, P.M. Stainless Steel 3D Laser Forming for Rapid Prototyping. In International Congress on Applications of Lasers & Electro-Optics; Laser Institute of America: Orlando, FL, USA, 2001; Volume 2001, pp. 808–816. [Google Scholar]
- Ambrogio, G.; De Napoli, L.; Filice, L.; Gagliardi, F.; Muzzupappa, M. Application of Incremental Forming process for high customised medical product manufacturing. J. Mater. Process. Technol. 2005, 162, 156–162. [Google Scholar] [CrossRef]
- Eksteen, P.D.; Van der Merwe, A.F. Incremental sheet forming (ISF) in the manufacturing of titanium based plate implants in the bio-medical sector. In Proceedings of the 42nd Computers and Industrial Engineering, Cape Town, South Africa, 16–18 July 2012; pp. 15–18. [Google Scholar]
- Calle, M.A.; Salmi, M.; Mazzariol, L.M.; Alves, M.; Kujala, P. Additive manufacturing of miniature marine structures for crashworthiness verification: Scaling technique and experimental tests. Mar. Struct. 2020, 72, 102764. [Google Scholar] [CrossRef]
- Kestilä, A.; Nordling, K.; Miikkulainen, V.; Kaipio, M.; Tikka, T.; Salmi, M.; Auer, A.; Leskelä, M.; Ritala, M. Towards space-grade 3D-printed, ALD-coated small satellite propulsion components for fluidics. Addit. Manuf. 2018, 22, 31–37. [Google Scholar] [CrossRef]
- Delic, M.; Eyers, D.R. The effect of additive manufacturing adoption on supply chain flexibility and performance: An empirical analysis from the automotive industry. Int. J. Prod. Econ. 2020, 228, 107689. [Google Scholar] [CrossRef]
- Kretzschmar, N.; Chekurov, S.; Salmi, M.; Tuomi, J. Evaluating the readiness level of additively manufactured digital spare parts: An industrial perspective. Appl. Sci. 2018, 8, 1837. [Google Scholar] [CrossRef] [Green Version]
- Gibson, I.; Srinath, A. Simplifying medical additive manufacturing: Making the surgeon the designer. Procedia Technol. 2015, 20, 237–242. [Google Scholar] [CrossRef] [Green Version]
- Conner, B.P.; Manogharan, G.P.; Martof, A.N.; Rodomsky, L.M.; Rodomsky, C.M.; Jordan, D.C.; Limperos, J.W. Making sense of 3-D printing: Creating a map of additive manufacturing products and services. Addit. Manuf. 2014, 1, 64–76. [Google Scholar] [CrossRef]
- Baumers, M.; Dickens, P.; Tuck, C.; Hague, R. The cost of additive manufacturing: Machine productivity, economies of scale and technology-push. Technol. Forecast. Soc. Chang. 2016, 102, 193–201. [Google Scholar] [CrossRef]
- Thomas, D.S.; Gilbert, S.W. Costs and cost effectiveness of additive manufacturing. Nist Spec. Publ. 2014, 1176, 12. [Google Scholar]
- Van Noort, R. The future of dental devices is digital. Dent. Mater. 2012, 28, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Jockusch, J.; Özcan, M. Additive manufacturing of dental polymers: An overview on processes, materials and applications. Dent. Mater. J. 2020, 39, 345–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghomi, E.R.; Khosravi, F.; Neisiany, R.E.; Singh, S.; Ramakrishna, S. Future of Additive Manufacturing in Healthcare. Curr. Opin. Biomed. Eng. 2020, 17, 100255. [Google Scholar] [CrossRef]
- Singh, S.; Ramakrishna, S. Biomedical applications of additive manufacturing: Present and future. Curr. Opin. Biomed. Eng. 2017, 2, 105–115. [Google Scholar] [CrossRef]
- Pettersson, A.; Salmi, M.; Vallittu, P.; Serlo, W.; Tuomi, J.; Mäkitie, A.A. Main Clinical Use of Additive Manufacturing (Three-Dimensional Printing) in Finland Restricted to the Head and Neck Area in 2016–2017. Scand. J. Surg. 2020, 109, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Zadpoor, A.A.; Malda, J. Additive Manufacturing of Biomaterials, Tissues, and Organs. Ann. Biomed. Eng. 2016, 1, 1–11. [Google Scholar] [CrossRef]
- Bibb, R.; Eggbeer, D.; Paterson, A. Medical Modelling: The Application of Advanced Design and Development Techniques in Medicine; Woodhead Publishing: Cambridge, UK, 2006. [Google Scholar]
- Kumbhar, N.N.; Mulay, A.V. Post processing methods used to improve surface finish of products which are manufactured by additive manufacturing technologies: A review. J. Inst. Eng. India Ser. C 2018, 99, 481–487. [Google Scholar] [CrossRef]
- Ballard, D.H.; Mills, P.; Duszak, R., Jr.; Weisman, J.A.; Rybicki, F.J.; Woodard, P.K. Medical 3D printing cost-Savings in Orthopedic and Maxillofacial Surgery: Cost analysis of operating room time saved with 3D printed anatomic models and surgical guides. Acad. Radiol. 2020, 27, 1103–1113. [Google Scholar] [CrossRef]
- Choonara, Y.E.; du Toit, L.C.; Kumar, P.; Kondiah, P.P.; Pillay, V. 3D-printing and the effect on medical costs: A new era? Expert Rev. Pharm. Outcomes Res. 2016, 16, 23–32. [Google Scholar] [CrossRef]
- Mahmoud, A.; Bennett, M. Introducing 3-dimensional printing of a human anatomic pathology specimen: Potential benefits for undergraduate and postgraduate education and anatomic pathology practice. Arch. Pathol. Lab. Med. 2015, 139, 1048–1051. [Google Scholar] [CrossRef]
- Tack, P.; Victor, J.; Gemmel, P.; Annemans, L. 3D-printing techniques in a medical setting: A systematic literature review. Biomed. Eng. Online 2016, 15, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballard, D.H.; Tappa, K.; Boyer, C.J.; Jammalamadaka, U.; Hemmanur, K.; Weisman, J.A.; Alexander, J.S.; Mills, D.K.; Woodard, P.K. Antibiotics in 3D-printed implants, instruments and materials: Benefits, challenges and future directions. J. 3d Print. Med. 2019, 3, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Fang, Y.; Liao, Y.; Chen, G.; Gao, C.; Zhu, P. 3D printing and digital processing techniques in dentistry: A review of literature. Adv. Eng. Mater. 2019, 21, 1801013. [Google Scholar] [CrossRef]
- Javaid, M.; Haleem, A. Current status and applications of additive manufacturing in dentistry: A literature-based review. J. Oral Biol. Craniofacial Res. 2019, 9, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Aho, J.; Bøtker, J.P.; Genina, N.; Edinger, M.; Arnfast, L.; Rantanen, J. Roadmap to 3D-printed oral pharmaceutical dosage forms: Feedstock filament properties and characterization for fused deposition modeling. J. Pharm. Sci. 2019, 108, 26–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salmi, M.; Paloheimo, K.; Tuomi, J.; Ingman, T.; Mäkitie, A. A digital process for additive manufacturing of occlusal splints: A clinical pilot study. J. R. Soc. Interface 2013, 10, 20130203. [Google Scholar] [CrossRef]
- Aquino, R.P.; Barile, S.; Grasso, A.; Saviano, M. Envisioning smart and sustainable healthcare: 3D Printing technologies for personalized medication. Futures 2018, 103, 35–50. [Google Scholar] [CrossRef]
- Javaid, M.; Haleem, A. Current status and challenges of Additive manufacturing in orthopaedics: An overview. J. Clin. Orthop. Trauma 2019, 10, 380–386. [Google Scholar] [CrossRef]
- Emelogu, A.; Marufuzzaman, M.; Thompson, S.M.; Shamsaei, N.; Bian, L. Additive manufacturing of biomedical implants: A feasibility assessment via supply-chain cost analysis. Addit. Manuf. 2016, 11, 97–113. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M. 3D printed medical parts with different materials using additive manufacturing. Clin. Epidemiol. Glob. Health 2020, 8, 215–223. [Google Scholar] [CrossRef] [Green Version]
- Murr, L.E.; Gaytan, S.M.; Medina, F.; Lopez, H.; Martinez, E.; Machado, B.I.; Hernandez, D.H.; Martinez, L.; Lopez, M.I.; Wicker, R.B. Next-generation biomedical implants using additive manufacturing of complex, cellular and functional mesh arrays. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 1999–2032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peltola, M.J.; Vallittu, P.K.; Vuorinen, V.; Aho, A.A.; Puntala, A.; Aitasalo, K.M. Novel composite implant in craniofacial bone reconstruction. Eur. Arch. Oto Rhino Laryngol. 2012, 269, 623–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramakrishnaiah, R.; Mohammad, A.; Divakar, D.D.; Kotha, S.B.; Celur, S.L.; Hashem, M.I.; Vallittu, P.K.; Rehman, I.U. Preliminary fabrication and characterization of electron beam melted Ti–6Al–4V customized dental implant. Saudi J. Biol. Sci. 2017, 24, 787–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazir, A.; Azhar, A.; Nazir, U.; Liu, Y.; Qureshi, W.S.; Chen, J.; Alanazi, E. The rise of 3D Printing entangled with smart computer aided design during COVID-19 era. J. Manuf. Syst. 2020. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Lin, S.; Xie, Q.; Ouyang, W.; Tan, T.; Li, J.; Chen, Z.; Yang, J.; Wu, H.; Pan, J. Impact of 3D printing technology on the comprehension of surgical liver anatomy. Surg. Endosc. 2019, 33, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Javaid, M.; Haleem, A. Additive manufacturing applications in medical cases: A literature based review. Alex. J. Med. 2018, 54, 411–422. [Google Scholar] [CrossRef] [Green Version]
- Tuomi, J.; Paloheimo, K.; Vehviläinen, J.; Björkstrand, R.; Salmi, M.; Huotilainen, E.; Kontio, R.; Rouse, S.; Gibson, I.; Mäkitie, A.A. A novel classification and online platform for planning and documentation of medical applications of additive manufacturing. Surg. Innov. 2014, 21, 553–559. [Google Scholar] [CrossRef]
- Tuomi, J.; Paloheimo, K.; Björkstrand, R.; Salmi, M.; Paloheimo, M.; Mäkitie, A.A. Medical applications of rapid prototyping—From applications to classification. In Innovative Developments in Design and Manufacturing—Advanced Research in Virtual and Rapid Prototyping; CRC Press: Boca Raton, FL, USA, 2010; pp. 701–704. [Google Scholar]
- Ventola, C.L. Medical applications for 3D printing: Current and projected uses. Pharm. Ther. 2014, 39, 704. [Google Scholar]
- Salmi, M. Possibilities of preoperative medical models made by 3D printing or additive manufacturing. J. Med. Eng. 2016, 2016, 6191526. [Google Scholar] [CrossRef] [Green Version]
- Bücking, T.M.; Hill, E.R.; Robertson, J.L.; Maneas, E.; Plumb, A.A.; Nikitichev, D.I. From medical imaging data to 3D printed anatomical models. PLoS ONE 2017, 12, e0178540. [Google Scholar] [CrossRef] [Green Version]
- Marro, A.; Bandukwala, T.; Mak, W. Three-dimensional printing and medical imaging: A review of the methods and applications. Curr. Probl. Diagn. Radiol. 2016, 45, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Salmi, M.; Tuomi, J.; Paloheimo, K.; Paloheimo, M.; Björkstrand, R.; Mäkitie, A.A.; Mesimäki, K.; Kontio, R. Digital design and rapid manufacturing in orbital wall reconstruction. In Innovative Developments in Design and Manufacturing—Advanced Research in Virtual and Rapid Prototyping; CRC Press: Boca Raton, FL, USA, 2010; pp. 339–342. [Google Scholar]
- Wang, X.; Xu, S.; Zhou, S.; Xu, W.; Leary, M.; Choong, P.; Qian, M.; Brandt, M.; Xie, Y.M. Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: A review. Biomaterials 2016, 83, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Tahayeri, A.; Morgan, M.; Fugolin, A.P.; Bompolaki, D.; Athirasala, A.; Pfeifer, C.S.; Ferracane, J.L.; Bertassoni, L.E. 3D printed versus conventionally cured provisional crown and bridge dental materials. Dent. Mater. 2018, 34, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Akmal, J.S.; Salmi, M.; Mäkitie, A.; Björkstrand, R.; Partanen, J. Implementation of industrial additive manufacturing: Intelligent implants and drug delivery systems. J. Funct. Biomater. 2018, 9, 41. [Google Scholar] [CrossRef] [Green Version]
- Prasad, L.K.; Smyth, H. 3D Printing technologies for drug delivery: A review. Drug Dev. Ind. Pharm. 2016, 42, 1019–1031. [Google Scholar] [CrossRef]
- Hieu, L.C.; Bohez, E.; Vander Sloten, J.; Phien, H.N.; Esichaikul, V.; Binh, P.H.; An, P.V.; To, N.C.; Oris, P. Design and manufacturing of personalized implants and standardized templates for cranioplasty applications. In Proceedings of the 2002 IEEE International Conference on Industrial Technology, IEEE ICIT’02, Bankok, Thailand, 11–14 December 2002; IEEE: New York, NY, USA, 2002; Volume 2, pp. 1025–1030. [Google Scholar]
- Hieu, L.C.; Bohez, E.; Vander Sloten, J.; Phien, H.N.; Vatcharaporn, E.; Binh, P.H.; An, P.V.; Oris, P. Design for medical rapid prototyping of cranioplasty implants. Rapid Prototyp. J. 2003, 9, 175–186. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, Q.; Li, X.; Zhao, C.; Quan, X.; Zhao, R.; Chen, Z.; Li, Y. Preliminary application of a multi-level 3D printing drill guide template for pedicle screw placement in severe and rigid scoliosis. Eur. Spine J. 2017, 26, 1684–1689. [Google Scholar] [CrossRef]
- Culmone, C.; Smit, G.; Breedveld, P. Additive manufacturing of medical instruments: A state-of-the-art review. Addit. Manuf. 2019, 27, 461–473. [Google Scholar] [CrossRef]
- Kontio, R.; Björkstrand, R.; Salmi, M.; Paloheimo, M.; Paloheimo, K.S.; Tuomi, J.; Mäkitie, A. Designing and additive manufacturing a prototype for a novel instrument for mandible fracture reduction. Surgery 2012, S1, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Chin, S.; Wilde, F.; Neuhaus, M.; Schramm, A.; Gellrich, N.; Rana, M. Accuracy of virtual surgical planning of orthognathic surgery with aid of CAD/CAM fabricated surgical splint—A novel 3D analyzing algorithm. J. Cranio Maxillofac. Surg. 2017, 45, 1962–1970. [Google Scholar] [CrossRef]
- Gibson, I.; Rosen, D.; Stucker, B. Direct digital manufacturing. In Additive Manufacturing Technologies; Springer: Berlin/Heidelberg, Germany, 2015; pp. 375–397. [Google Scholar]
- Herbert, N.; Simpson, D.; Spence, W.D.; Ion, W. A preliminary investigation into the development of 3-D printing of prosthetic sockets. J. Rehabil. Res. Dev. 2005, 42, 141. [Google Scholar] [CrossRef] [PubMed]
- Ten Kate, J.; Smit, G.; Breedveld, P. 3D-printed upper limb prostheses: A review. Disabil. Rehabil. Assist. Technol. 2017, 12, 300–314. [Google Scholar] [CrossRef] [PubMed]
- Paterson, A.M.; Donnison, E.; Bibb, R.J.; Ian Campbell, R. Computer-aided design to support fabrication of wrist splints using 3D printing: A feasibility study. Hand Ther. 2014, 19, 102–113. [Google Scholar] [CrossRef] [Green Version]
- Rosicky, J.; Grygar, A.; Chapcak, P.; Bouma, T.; Rosicky, J. Application of 3D scanning in prosthetic & orthotic clinical practice. In Proceedings of the 7th International Conference on 3D Body Scanning Technologies, Lugano, Switzerland, 30 November–1 December 2016; pp. 88–97. [Google Scholar]
- Bártolo, P.J.; Chua, C.K.; Almeida, H.A.; Chou, S.M.; Lim, A. Biomanufacturing for tissue engineering: Present and future trends. Virtual Phys. Prototyp. 2009, 4, 203–216. [Google Scholar] [CrossRef]
- Bidanda, B.; Bártolo, P.J. Virtual Prototyping & Bio Manufacturing in Medical Applications; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Danna, N.R.; Leucht, P. Designing Resorbable Scaffolds for Bone Defects. Bull. Nyu Hosp. Jt. Dis. 2019, 77, 39–44. [Google Scholar]
- Zadpoor, A.A. Design for additive bio-manufacturing: From patient-specific medical devices to rationally designed meta-biomaterials. Int. J. Mol. Sci. 2017, 18, 1607. [Google Scholar] [CrossRef] [Green Version]
- Lowther, M.; Louth, S.; Davey, A.; Hussain, A.; Ginestra, P.; Carter, L.; Eisenstein, N.; Grover, L.; Cox, S. Clinical, industrial, and research perspectives on powder bed fusion additively manufactured metal implants. Addit. Manuf. 2019, 28, 565–584. [Google Scholar] [CrossRef] [Green Version]
- Tappa, K.; Jammalamadaka, U. Novel biomaterials used in medical 3D printing techniques. J. Funct. Biomater. 2018, 9, 17. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Ho, C.; Zhang, C.; Wang, B. A review on the 3D printing of functional structures for medical phantoms and regenerated tissue and organ applications. Engineering 2017, 3, 653–662. [Google Scholar] [CrossRef]
- Alku, P.; Murtola, T.; Malinen, J.; Kuortti, J.; Story, B.; Airaksinen, M.; Salmi, M.; Vilkman, E.; Geneid, A. OPENGLOT–An open environment for the evaluation of glottal inverse filtering. Speech Commun. 2019, 107, 38–47. [Google Scholar] [CrossRef]
- Mäkitie, A.A.; Salmi, M.; Lindford, A.; Tuomi, J.; Lassus, P. Three-dimensional printing for restoration of the donor face: A new digital technique tested and used in the first facial allotransplantation patient in Finland. J. Plast. Reconstr. Aesthetic Surg. 2016, 69, 1648–1652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szymor, P.; Kozakiewicz, M.; Olszewski, R. Accuracy of open-source software segmentation and paper-based printed three-dimensional models. J. Cranio Maxillofac. Surg. 2016, 44, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Mäkitie, A.; Paloheimo, K.S.; Björkstrand, R.; Salmi, M.; Kontio, R.; Salo, J.; Yan, Y.; Paloheimo, M.; Tuomi, J. Medical applications of rapid prototyping--three-dimensional bodies for planning and implementation of treatment and for tissue replacement. Duodecim 2010, 126, 143–151. [Google Scholar] [PubMed]
- Salmi, M.; Paloheimo, K.; Tuomi, J.; Wolff, J.; Mäkitie, A. Accuracy of medical models made by additive manufacturing (rapid manufacturing). J. Cranio Maxillofac. Surg. 2013, 41, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Ionita, C.N.; Mokin, M.; Varble, N.; Bednarek, D.R.; Xiang, J.; Snyder, K.V.; Siddiqui, A.H.; Levy, E.I.; Meng, H.; Rudin, S. Challenges and limitations of patient-specific vascular phantom fabrication using 3D Polyjet printing. In Medical Imaging 2014: Biomedical Applications in Molecular, Structural, and Functional Imaging; International Society for Optics and Photonics: Washington, DC, USA, 2014; Volume 9038, p. 90380M. [Google Scholar]
- Aitasalo, K.M.; Piitulainen, J.M.; Rekola, J.; Vallittu, P.K. Craniofacial bone reconstruction with bioactive fiber-reinforced composite implant. Head Neck 2014, 36, 722–728. [Google Scholar] [CrossRef]
- Bergstroem, J.S.; Hayman, D. An overview of mechanical properties and material modeling of polylactide (PLA) for medical applications. Ann. Biomed. Eng. 2016, 44, 330–340. [Google Scholar] [CrossRef]
- Panesar, S.S.; Magnetta, M.; Mukherjee, D.; Abhinav, K.; Branstetter, B.F.; Gardner, P.A.; Iv, M.; Fernandez-Miranda, J.C. Patient-specific 3-dimensionally printed models for neurosurgical planning and education. Neurosurg. Focus 2019, 47, E12. [Google Scholar] [CrossRef] [Green Version]
- Wheat, E.; Vlasea, M.; Hinebaugh, J.; Metcalfe, C. Sinter structure analysis of titanium structures fabricated via binder jetting additive manufacturing. Mater. Des. 2018, 156, 167–183. [Google Scholar] [CrossRef]
- Akmal, J.S.; Salmi, M.; Hemming, B.; Linus, T.; Suomalainen, A.; Kortesniemi, M.; Partanen, J.; Lassila, A. Cumulative inaccuracies in implementation of additive manufacturing through medical imaging, 3D thresholding, and 3D modeling: A case study for an end-use implant. Appl. Sci. 2020, 10, 2968. [Google Scholar] [CrossRef]
- Tuomi, J.T.; Björkstrand, R.V.; Pernu, M.L.; Salmi, M.V.; Huotilainen, E.I.; Wolff, J.E.; Vallittu, P.K.; Mäkitie, A.A. In vitro cytotoxicity and surface topography evaluation of additive manufacturing titanium implant materials. J. Mater. Sci. Mater. Med. 2017, 28, 53. [Google Scholar] [CrossRef] [Green Version]
- Balažic, M.; Recek, D.; Kramar, D.; Milfelner, M.; Kopač, J. Development process and manufactu-ring of modern medical implants with LENS technology. J. Achiev. Mater. Manuf. Eng. 2009, 32, 46–52. [Google Scholar]
- Honigmann, P.; Sharma, N.; Okolo, B.; Popp, U.; Msallem, B.; Thieringer, F.M. Patient-specific surgical implants made of 3D printed PEEK: Material, technology, and scope of surgical application. Biomed Res. Int. 2018, 2018, 4520636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwarzer, E.; Holtzhausen, S.; Scheithauer, U.; Ortmann, C.; Oberbach, T.; Moritz, T.; Michaelis, A. Process development for additive manufacturing of functionally graded alumina toughened zirconia components intended for medical implant application. J. Eur. Ceram. Soc. 2019, 39, 522–530. [Google Scholar] [CrossRef]
- Igawa, K.; Mochizuki, M.; Sugimori, O.; Shimizu, K.; Yamazawa, K.; Kawaguchi, H.; Nakamura, K.; Takato, T.; Nishimura, R.; Suzuki, S. Tailor-made tricalcium phosphate bone implant directly fabricated by a three-dimensional ink-jet printer. J. Artif. Organs 2006, 9, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Honigmann, P.; Cao, S.; Thieringer, F. Dimensional characteristics of FDM 3D printed PEEK implant for craniofacial reconstructions. Trans. Addit. Manuf. Meets Med. 2020, 2. [Google Scholar] [CrossRef]
- Jardini, A.L.; Larosa, M.A.; Macedo, M.F.; Bernardes, L.F.; Lambert, C.S.; Zavaglia, C.; Maciel Filho, R.; Calderoni, D.R.; Ghizoni, E.; Kharmandayan, P. Improvement in cranioplasty: Advanced prosthesis biomanufacturing. Procedia Cirp 2016, 49, 203–208. [Google Scholar] [CrossRef] [Green Version]
- Wilkes, J.; Hagedorn, Y.; Meiners, W.; Wissenbach, K. Additive manufacturing of ZrO2-Al2O3 ceramic components by selective laser melting. Rapid Prototyp. J. 2013, 19, 51–57. [Google Scholar] [CrossRef]
- Kukko, K.; Akmal, J.S.; Kangas, A.; Salmi, M.; Björkstrand, R.; Viitanen, A.-K.; Partanen, J.; Joshua, M. Pearce Additively Manufactured Parametric Universal Clip-System: An Open Source Approach for Aiding Personal Exposure Measurement in the Breathing Zone. Appl. Sci. 2020, 10, 6671. [Google Scholar] [CrossRef]
- Salmi, M.; Tuomi, J.; Sirkkanen, R.; Ingman, T.; Makitie, A. Rapid tooling method for soft customized removable oral appliances. Open Dent. J. 2012, 6, 85–89. [Google Scholar] [CrossRef]
- Salmi, M.; Akmal, J.S.; Pei, E.; Wolff, J.; Jaribion, A.; Khajavi, S.H. 3D Printing in COVID-19: Productivity Estimation of the Most Promising Open Source Solutions in Emergency Situations. Appl. Sci. 2020, 10, 4004. [Google Scholar] [CrossRef]
- Tiwary, V.K.; Arunkumar, P.; Deshpande, A.S.; Rangaswamy, N. Surface enhancement of FDM patterns to be used in rapid investment casting for making medical implants. Rapid Prototyp. J. 2019, 25, 904–914. [Google Scholar] [CrossRef]
- Cloonan, A.J.; Shahmirzadi, D.; Li, R.X.; Doyle, B.J.; Konofagou, E.E.; McGloughlin, T.M. 3D-printed tissue-mimicking phantoms for medical imaging and computational validation applications. 3D Print. Addit. Manuf. 2014, 1, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Cao, S.; Msallem, B.; Kunz, C.; Brantner, P.; Honigmann, P.; Thieringer, F.M. Effects of Steam Sterilization on 3D Printed Biocompatible Resin Materials for Surgical Guides—An Accuracy Assessment Study. J. Clin. Med. 2020, 9, 1506. [Google Scholar] [CrossRef] [PubMed]
- Jahnke, P.; Schwarz, S.; Ziegert, M.; Schwarz, F.B.; Hamm, B.; Scheel, M. Paper-based 3D printing of anthropomorphic CT phantoms: Feasibility of two construction techniques. Eur. Radiol. 2018, 29, 1384–1390. [Google Scholar] [CrossRef] [PubMed]
- Väyrynen, V.O.; Tanner, J.; Vallittu, P.K. The anisotropicity of the flexural properties of an occlusal device material processed by stereolithography. J. Prosthet. Dent. 2016, 116, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Huotilainen, E.; Salmi, M.; Lindahl, J. Three-dimensional printed surgical templates for fresh cadaveric osteochondral allograft surgery with dimension verification by multivariate computed tomography analysis. Knee 2019, 26, 923–932. [Google Scholar] [CrossRef]
- Cascón, W.P.; Revilla-León, M. Digital workflow for the design and additively manufacture of a splinted framework and custom tray for the impression of multiple implants: A dental technique. J. Prosthet. Dent. 2018, 120, 805–811. [Google Scholar] [CrossRef]
- Lathers, S.; La Belle, J. Advanced manufactured fused filament fabrication 3D printed osseointegrated prosthesis for a transhumeral amputation using Taulman 680 FDA. 3D Print. Addit. Manuf. 2016, 3, 166–174. [Google Scholar] [CrossRef]
- Freeman, D.; Wontorcik, L. Stereolithography and prosthetic test socket manufacture: A cost/benefit analysis. JPO J. Prosthet. Orthot. 1998, 10, 17–20. [Google Scholar] [CrossRef]
- Sengeh, D.M. Advanced Prototyping of Variable Impedance Prosthetic Sockets For Trans-Tibial Amputees: Polyjet Matrix 3D Printing of Comfortable Prosthetic Sockets Using Digital Anatomical Data. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2012. [Google Scholar]
- Mohseni, M.; Bas, O.; Castro, N.J.; Schmutz, B.; Hutmacher, D.W. Additive biomanufacturing of scaffolds for breast reconstruction. Addit. Manuf. 2019, 30, 100845. [Google Scholar] [CrossRef]
- Poh, P.S.; Chhaya, M.P.; Wunner, F.M.; De-Juan-Pardo, E.M.; Schilling, A.F.; Schantz, J.; van Griensven, M.; Hutmacher, D.W. Polylactides in additive biomanufacturing. Adv. Drug Deliv. Rev. 2016, 107, 228–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, S.; Han, J.; Sharma, N.; Msallem, B.; Jeong, W.; Son, J.; Kunz, C.; Kang, H.; Thieringer, F.M. In Vitro Mechanical and Biological Properties of 3D Printed Polymer Composite and β-Tricalcium Phosphate Scaffold on Human Dental Pulp Stem Cells. Materials 2020, 13, 3057. [Google Scholar] [CrossRef]
- Inzana, J.A.; Olvera, D.; Fuller, S.M.; Kelly, J.P.; Graeve, O.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials 2014, 35, 4026–4034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schult, M.; Buckow, E.; Seitz, H. Experimental studies on 3D printing of barium titanate ceramics for medical applications. Curr. Dir. Biomed. Eng. 2016, 2, 95–99. [Google Scholar] [CrossRef]
- Sahu, K.K.; Modi, Y.K. Investigation on dimensional accuracy, compressive strength and measured porosity of additively manufactured calcium sulphate porous bone scaffolds. Mater. Technol. 2020, 1–12. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M.; Saxena, A. Additive manufacturing applications in cardiology: A review. Egypt. Heart J. 2018, 70, 433–441. [Google Scholar] [CrossRef]
- Thompson, A.; McNally, D.; Maskery, I.; Leach, R.K. X-ray computed tomography and additive manufacturing in medicine: A review. Int. J. Metrol. Qual. Eng. 2017, 8, 17. [Google Scholar] [CrossRef] [Green Version]
- Calignano, F.; Galati, M.; Iuliano, L.; Minetola, P. Design of additively manufactured structures for biomedical applications: A review of the additive manufacturing processes applied to the biomedical sector. J. Healthc. Eng. 2019, 2019, 9748212. [Google Scholar] [CrossRef]
- Galante, R.; Figueiredo-Pina, C.G.; Serro, A.P. Additive manufacturing of ceramics for dental applications: A review. Dent. Mater. 2019, 35, 825–846. [Google Scholar] [CrossRef]
- Ramola, M.; Yadav, V.; Jain, R. On the adoption of additive manufacturing in healthcare: A literature review. J. Manuf. Technol. Manag. 2019, 30, 48–69. [Google Scholar] [CrossRef]
- Bhargav, A.; Sanjairaj, V.; Rosa, V.; Feng, L.W.; Fuh, Y.H.J. Applications of additive manufacturing in dentistry: A review. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2018, 106, 2058–2064. [Google Scholar] [CrossRef] [PubMed]
- Revilla-León, M.; Sadeghpour, M.; Özcan, M. A Review of the Applications of Additive Manufacturing Technologies Used to Fabricate Metals in Implant Dentistry. J. Prosthodont. 2020, 29, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Revilla-León, M.; Meyer, M.J.; Özcan, M. Metal additive manufacturing technologies: Literature review of current status and prosthodontic applications. Int. J. Comput. Dent. 2019, 22, 55–67. [Google Scholar] [PubMed]
- Trivedi, M.; Jee, J.; Silva, S.; Blomgren, C.; Pontinha, V.M.; Dixon, D.L.; Van Tassel, B.; Bortner, M.J.; Williams, C.; Gilmer, E. Additive manufacturing of pharmaceuticals for precision medicine applications: A review of the promises and perils in implementation. Addit. Manuf. 2018, 23, 319–328. [Google Scholar] [CrossRef]
- Oh, W.J.; Lee, W.J.; Kim, M.S.; Jeon, J.B.; Shim, D.S. Repairing additive-manufactured 316L stainless steel using direct energy deposition. Opt. Laser Technol. 2019, 117, 6–17. [Google Scholar] [CrossRef]
- Olszewski, R.; Tilleux, C.; Hastir, J.; Delvaux, L.; Danse, E. Holding Eternity in One’s Hand: First Three-Dimensional Reconstruction and Printing of the Heart from 2700 Years-Old Egyptian Mummy. Anat. Rec. 2019, 302, 912–916. [Google Scholar] [CrossRef]
- Ward, A.A.; Cordero, Z.C. Junction growth and interdiffusion during ultrasonic additive manufacturing of multi-material laminates. Scr. Mater. 2020, 177, 101–105. [Google Scholar] [CrossRef]
- Kaza, A.; Rembalsky, J.; Roma, N.; Yellapu, V.; Delong, W.G.; Stawicki, S.P. Medical applications of stereolithography: An overview. Int. J. Acad. Med. 2018, 4, 252. [Google Scholar]
- Feuerbach, T.; Kock, S.; Thommes, M. Characterisation of fused deposition modeling 3D printers for pharmaceutical and medical applications. Pharm. Dev. Technol. 2018, 23, 1136–1145. [Google Scholar] [CrossRef]
- Ait-Mansour, I.; Kretzschmar, N.; Chekurov, S.; Salmi, M.; Rech, J. Design-dependent shrinkage compensation modeling and mechanical property targeting of metal FFF. Prog. Addit. Manuf. 2020, 5, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Adumitroaie, A.; Antonov, F.; Khaziev, A.; Azarov, A.; Golubev, M.; Vasiliev, V.V. Novel Continuous Fiber Bi-Matrix Composite 3-D Printing Technology. Materials 2019, 12, 3011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, M.A.; Mykulowycz, N.M.; Shim, J.; Fontana, R.; Schmitt, P.; Roberts, A.; Ketkaew, J.; Shao, L.; Chen, W.; Bordeenithikasem, P. 3D printing metals like thermoplastics: Fused filament fabrication of metallic glasses. Mater. Today 2018, 21, 697–702. [Google Scholar] [CrossRef]
- Wolff, M.; Mesterknecht, T.; Bals, A.; Ebel, T.; Willumeit-Römer, R. FFF of Mg-Alloys for Biomedical Application. In Magnesium Technology 2019; Springer: Berlin/Heidelberg, Germany, 2019; pp. 43–49. [Google Scholar]
- Spencer, S.R.; Watts, L.K. Three-Dimensional Printing in Medical and Allied Health Practice: A Literature Review. J. Med. Imaging Radiat. Sci. 2020, 51, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Miyanaji, H.; Rahman, K.M.; Da, M.; Williams, C.B. Effect of fine powder particles on quality of binder jetting parts. Addit. Manuf. 2020, 36, 101587. [Google Scholar] [CrossRef]
- Huang, S.; Ye, C.; Zhao, H.; Fan, Z. Parameters optimization of binder jetting process using modified silicate as a binder. Mater. Manuf. Process. 2020, 35, 214–220. [Google Scholar] [CrossRef]
- Ziaee, M.; Crane, N.B. Binder jetting: A review of process, materials, and methods. Addit. Manuf. 2019, 28, 781–801. [Google Scholar] [CrossRef]
- Chhaya, M.P.; Poh, P.S.; Balmayor, E.R.; van Griensven, M.; Schantz, J.; Hutmacher, D.W. Additive manufacturing in biomedical sciences and the need for definitions and norms. Expert Rev. Med. Devices 2015, 12, 537–543. [Google Scholar] [CrossRef]







| Reference | Findings | Area |
|---|---|---|
| Ballard et al. [22] | cost and time savings | Orthopedic and maxillofacial surgery |
| Choonora et al. [23] | personalization | Transplants |
| Mahmoud et al. [24] | cost savings | Pathology specimens for students |
| Tack et al. [25] | time savings, improved medical outcome, decreased radiation exposure | Surgery |
| Ballard et al. [26] | incorporation of antibiotics | Implants |
| Lin et al. [27] | personalization, cost savings | Dental |
| Javaid et al. [28] | cost and time savings, personalization, digital storage | Dental |
| Aho et al. [29] | personalization | Pharmacy |
| Salmi et al. [30] | reduction of manual work | Dental appliances |
| Aquino et al. [31] | personalization, on-demand manufacturing | Pharmacy |
| Javaid et al. [32] | accuracy, cost and time savings, personalization, fully automated and digitized manufacturing | Orthopedics |
| Emelogu et al. [33] | supply chain possibilities | Implants |
| Gibson et al. [10] | surgeon as designer, innovation potential | Surgery |
| Haleem et al. [34] | ability to use different materials | Medical |
| Murr et al. [35] | ability to make complex geometries | Implants |
| Peltola et al. [36] | template for forming implants | Implants |
| Ramakrishnaiah et al. [37] | rough and porous surface texture, better stabilization and osseointegration | Dental implants |
| Nazir et al. [38] | design iterations, supply chain possibilities, complex geometries | Medical devices |
| Yang et al. [39] | improved understanding of anatomy and accuracy of surgery | Surgery |
| AM Process | Short Description | Material Form | Plastics | Metals | Ceramics | Trade/Other Names |
|---|---|---|---|---|---|---|
| Powder bed fusion (PBF) | thermal energy fuses regions of a powder bed | powder | +++ | +++ | + | selective laser sintering (SLS), direct metal laser sintering (DMLS), selective laser melting (SLM) |
| Material extrusion (MEX) | material dispensed through a nozzle | filament, pellets, paste | +++ | ++ | ++ | fused deposition modeling (FDM), (fused filament fabrication) FFF |
| VAT photo-polymerization (VP) | liquid photopolymer in a vat is cured by light | liquid | +++ | + | ++ | SLA, digital light projection (DLP) |
| Material jetting (MJ) | droplets of material are selectively deposited | liquid | +++ | + | + | PolyJet, NJP |
| Binder jetting (BJ) | a liquid bonding agent is selectively deposited | powder | +++ | ++ | + | 3D printing (3DP), ColorJet printing (CJP) |
| Sheet lamination (SL) | sheets of material are bonded | sheets | ++ | ++ | - | laminated object manufacturing (LOM), ultrasonic additive manufacturing (UAM) |
| Directed energy deposition (DED) | focused thermal energy used to fuse materials by melting when depositing | powder, wire | - | +++ | + | laser-engineered net shaping (LENS), EBAM |
| AM Process | Application | and Process Term | or Manufacturer |
|---|---|---|---|
| PBF | medical or dental or implants or surgery or clinical | powder bed fusion or PFB or selective laser sintering or SLS or direct laser sintering or DMLS | - |
| MEX | material extrusion or fused filament fabrication or FFF or fused deposition modeling of FDM | - | |
| VP | VAT photopolymerization or stereolithography or SLA | - | |
| MJ | material jetting or Polyjet or nano particle jetting | Objet | |
| BJ | binder jetting or Colorjet printing | Zcorp or Zprinter | |
| SL | sheet lamination or LOM or laminated object manufacturing | Mcor or Fabrisonic | |
| DED | directed energy deposition or DED or laser engineered net shaping or LENS | - |
| Application Area | PBF | MEX | VP | MJ | BJ | SL | DED |
|---|---|---|---|---|---|---|---|
| Medical models [44,50,70,71,72,73,74,75,76,77,78,79] | PA, PP | ABS+, PLA | Photocurable resin | VeroWhite, VeroClear, TangoPlus, Multi-material | ZP150, ZP151, PMMA | Paper | |
| Implants [37,47,49,50,67,80,81,82,83,84,85,86,87,88] | Ti6Al4VTi64, Co–Cr–Mo, Al2O3–ZrO2 | PEEK | Clear resin V4, ATZ, NextDent C&B | ZP150, TCP, nickel-based alloy 625, Titanium | Ti6Al4V | ||
| Tools, instruments and parts for medical devices [54,55,56,89,90,91,92,93,94,95,96] | PA, Co–Cr, Ti | ABS, ABS+, PLA, | ProtoGen O-XT 18420, Dental SG, Dental LT, Clear resin V2, Photocurable resin WaterShed XC 11122 | TangoPlus, HeartPrint Flex, MED610 | Paper | ||
| Medical aids, supportive guides, splints and prostheses [60,61,97,98,99,100,101] | PA | ABS, PLA, Nylon | Clear resin, Ciba–Geigy 5170, Somos 6110, Epoxy | Multi-material, Full Cure 720, ABS like, VeroWhite | ZP151, Stainless steel | ||
| Biomanufacturing [63,102,103,104,105,106,107] | PLA, PLGA | PCL, PLA, PLGA, TCP | PDLLA, HA | VisiJet PXL, Calcium phosphate, barium titanate |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salmi, M. Additive Manufacturing Processes in Medical Applications. Materials 2021, 14, 191. https://doi.org/10.3390/ma14010191
Salmi M. Additive Manufacturing Processes in Medical Applications. Materials. 2021; 14(1):191. https://doi.org/10.3390/ma14010191
Chicago/Turabian StyleSalmi, Mika. 2021. "Additive Manufacturing Processes in Medical Applications" Materials 14, no. 1: 191. https://doi.org/10.3390/ma14010191