Synthesis and Performance of TiO2/Fly Ash Cenospheres as a Catalytic Film in a Novel Type of Periodic Air-Sparged Photocatalytic Reactor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
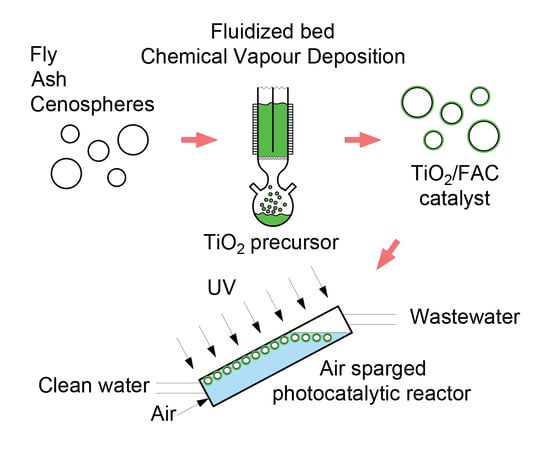
2.2. Catalyst Preparation
2.3. Characterisation of Materials
2.4. Photocatalytic Experiments
2.5. Process of Catalytic Film Formation and Breaking
2.6. Kinetic Calculation
3. Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kanki, T.; Hamasaki, S.; Sano, N.; Toyoda, A.; Hirano, K. Water purification by fluidized bed photocatalytic reactor using TiO2-Coated ceramic particles. Chem. Eng. J. 2005, 108, 155–160. [Google Scholar] [CrossRef]
- Chen, Q.; Ji, F.; Guo, Q.; Fan, J.; Xu, X. Combination of heterogeneous Fenton-like reaction and photocatalysis using Co-TiO2 nanocatalyst for activation of KHSO5 with visible light irradiation at ambient conditions. J. Environ. Sci. 2014, 26, 2440–2450. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Rahmawati, F.; Putri, F.R.; Masykur, A. The Photocatalytic Activity of Zns-TiO2 on a Carbon Fiber Prepared by Chemical Bath Deposition. Open Chem. 2019, 17, 132–141. [Google Scholar] [CrossRef]
- Huo, P.; Yan, Y.; Li, S.; Li, H.; Huang, W. Preparation and characterization of Cobalt Sulfophthalocyanine/TiO2/fly-ash cenospheres photocatalyst and study on degradation activity under visible light. Appl. Surf. Sci. 2009, 255, 6914–6917. [Google Scholar] [CrossRef]
- Huo, P.; Yan, Y.; Li, S.; Li, H.; Huang, W. Floating photocatalysts of fly-ash cenospheres supported AgCl/TiO2 films with enhanced Rhodamine B photodecomposition activity. Desalination 2010, 256, 196–200. [Google Scholar] [CrossRef]
- Wang, B.; Li, Q.; Wang, W.; Li, Y.; Zhai, J. Preparation and characterization of Fe3+-doped TiO2 on fly ash cenospheres for photocatalytic application. Appl. Surf. Sci. 2011, 257, 3473–3479. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, J.; Guo, X.; Ge, J.; Wu, H. Preparation of α-Fe2O3-TiO2/fly ash cenospheres photocatalyst and its mechanism of photocatalytic degradation. Colloids Surf. A Physicochem. Eng. Asp. 2015, 484, 434–440. [Google Scholar] [CrossRef]
- Wu, D.; Huo, P.; Lu, Z.; Gao, X.; Liu, X.; Shi, W.; Yan, Y. Preparation of heteropolyacid/TiO2/fly-ash-cenosphere photocatalyst for the degradation of ciprofloxacin from aqueous solutions. Appl. Surf. Sci. 2012, 258, 7008–7015. [Google Scholar] [CrossRef]
- Fan, H.; Chen, D.; Ai, X.; Han, S.; Wei, M.; Yang, L.; Liu, H.; Yang, J. Mesoporous TiO2 coated ZnFe2O4 nanocomposite loading on activated fly ash cenosphere for visible light photocatalysis. RSC Adv. 2018, 8, 1398–1406. [Google Scholar] [CrossRef] [Green Version]
- Bousselmi, L.; Geissen, S.U.; Schroeder, H. Textile wastewater treatment and reuse by solar catalysis: Results from a pilot plant in Tunisia. Water Sci. Technol. 2004, 49, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Zayani, G.; Bousselmi, L.; Mhenni, F.; Ghrabi, A. Solar photocatalytic degradation of commercial textile azo dyes: Performance of pilot plant scale thin film fixed-bed reactor. Desalination 2009, 246, 344–352. [Google Scholar] [CrossRef]
- Kumar, J.; Bansal, A. CFD simulations of immobilized-titanium dioxide based annular photocatalytic reactor: Model development and experimental validation. Indian J. Chem. Technol. 2015, 22, 95–104. [Google Scholar]
- Casado, C.; Marugán, J.; Timmers, R.; Muñoz, M.; van Grieken, R. Comprehensive multiphysics modeling of photocatalytic processes by computational fluid dynamics based on intrinsic kinetic parameters determined in a differential photoreactor. Chem. Eng. J. 2017, 310, 368–380. [Google Scholar] [CrossRef] [Green Version]
- Tolosana-Moranchel, A.; Casas, J.A.; Carbajo, J.; Faraldos, M.; Bahamonde, A. Influence of TiO2 optical parameters in a slurry photocatalytic reactor: Kinetic modelling. Appl. Catal. B Environ. 2017, 200, 164–173. [Google Scholar] [CrossRef]
- Van Gerven, T.; Mul, G.; Moulijn, J.; Stankiewicz, A. A review of intensification of photocatalytic processes. Chem. Eng. Process. 2007, 46, 781–789. [Google Scholar] [CrossRef]
- Abdel-Maksoud, Y.; Imam, E.; Ramadan, A. TiO2 solar photocatalytic reactor systems: Selection of reactor design for scale-up and commercialization—Analytical review. Catalysts 2016, 6, 26. [Google Scholar] [CrossRef] [Green Version]
- Fendrich, M.A.; Quaranta, A.; Orlandi, M.; Bettonte, M.; Miotello, A. Solar concentration for wastewaters remediation: A review of materials and technologies. Appl. Sci. 2019, 9, 26. [Google Scholar] [CrossRef] [Green Version]
- Xing, Z.; Zhang, J.; Cui, J.; Yin, J.; Zhao, T.; Kuang, J.; Xiu, Z.; Wan, N.; Zhou, W. Recent advances in floating TiO2-based photocatalysts for environmental application. Appl. Catal. B Environ. 2018, 225, 452–467. [Google Scholar] [CrossRef]
- Berkowicz, G.; Bradło, D.; Żukowski, W. Cenospheres as an innovative fluidised bed material. Czas. Tech. Chem. 2016, 113, 3–10. [Google Scholar]
- Reilly, K.; Fang, B.; Taghipour, F.; Wilkinson, D.P. Enhanced photocatalytic hydrogen production in a UV-irradiated fluidized bed reactor. J. Catal. 2017, 353, 63–73. [Google Scholar] [CrossRef]
- Spasiano, D.; Marotta, R.; Malato, S.; Fernandez-Ibañez, P.; Di Somma, I. Solar photocatalysis: Materials, reactors, some commercial, and pre-industrialized applications. A comprehensive approach. Appl. Catal. B Environ. 2015, 170–171, 90–123. [Google Scholar] [CrossRef]
- Jung, C.; Funkena, K.-H.; Ortnerb, J. PROPHIS: Parabolic trough-facility for organic photochemical syntheses in sunlight. Photochem. Photobiol. Sci. 2005, 4, 409–411. [Google Scholar] [CrossRef] [PubMed]
- Castilla-Caballero, D.; Machuca-Martínez, F.; Bustillo-Lecompte, C.; Colina-Márquez, J. Photocatalytic degradation of commercial acetaminophen: Evaluation, modeling, and scaling-up of photoreactors. Catalysts 2018, 8, 15. [Google Scholar] [CrossRef] [Green Version]
- Dariani, R.S.; Esmaeili, A.; Mortezaali, A.; Dehghanpour, S. Photocatalytic reaction and degradation of methylene blue on TiO2 nano-sized particles. Optik 2016, 127, 7143–7154. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.M. Photocatalytic degradation pathway of methylene blue in water. Appl. Catal. B Environ. 2001, 31, 145–157. [Google Scholar] [CrossRef]
- Dulian, P.; Nachit, W.; Jaglarz, J.; Zięba, P.; Kanak, J.; Żukowski, W. Photocatalytic methylene blue degradation on multilayer transparent TiO2 coatings. Opt. Mater. 2019, 90, 264–272. [Google Scholar] [CrossRef]










| Mass of the Catalyst (g) | Mixing Mode | k (1/min) | R2 | (min × g) | U (%) | |
|---|---|---|---|---|---|---|
| 2 | 10/0 | 0.00902 | 0.996 | 77 | 154 | 75 |
| 10/10 | 0.00999 | 0.996 | 69 | 139 | 81 | |
| 10/20 | 0.00696 | 0.997 | 100 | 199 | 77 | |
| 1 | 10/0 | 0.00604 | 0.987 | 115 | 115 | 100 |
| 10/10 | 0.00619 | 0.992 | 112 | 112 | 100 | |
| 10/20 | 0.00450 | 0.979 | 154 | 154 | 100 | |
| 0.5 | 10/0 | 0.00093 | 0.977 | 748 | 374 | 31 |
| 10/10 | 0.00287 | 0.997 | 242 | 121 | 93 | |
| 10/20 | 0.00275 | 0.980 | 252 | 126 | 122 | |
| photolysis | 10/0 | 0.00055* | 0.947 | 1251 | — | — |
| 10/10 | 0.00056* | 0.976 | 1239 | — | — | |
| 10/20 | 0.00043* | 0.852 | 1608 | — | — |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żukowski, W.; Migas, P.; Bradło, D.; Dulian, P. Synthesis and Performance of TiO2/Fly Ash Cenospheres as a Catalytic Film in a Novel Type of Periodic Air-Sparged Photocatalytic Reactor. Materials 2020, 13, 1691. https://doi.org/10.3390/ma13071691
Żukowski W, Migas P, Bradło D, Dulian P. Synthesis and Performance of TiO2/Fly Ash Cenospheres as a Catalytic Film in a Novel Type of Periodic Air-Sparged Photocatalytic Reactor. Materials. 2020; 13(7):1691. https://doi.org/10.3390/ma13071691
Chicago/Turabian StyleŻukowski, Witold, Przemysław Migas, Dariusz Bradło, and Piotr Dulian. 2020. "Synthesis and Performance of TiO2/Fly Ash Cenospheres as a Catalytic Film in a Novel Type of Periodic Air-Sparged Photocatalytic Reactor" Materials 13, no. 7: 1691. https://doi.org/10.3390/ma13071691