Liquid Phase Hydrogenation of Pharmaceutical Interest Nitroarenes over Gold-Supported Alumina Nanowires Catalysts
Abstract
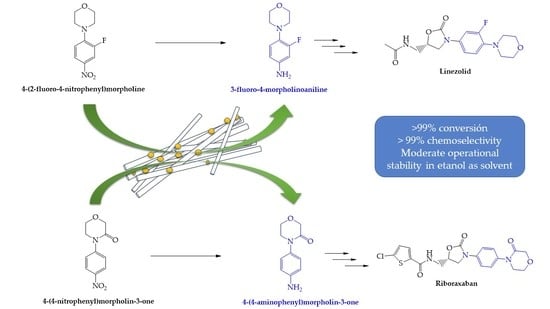
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the Substrates
2.2.1. Synthesis of 4-(2-fluoro-4-nitrophenyl)morpholine
2.2.2. Synthesis of 4-(4-nitrophenyl)morpholin-3-one
2.3. Al2O3 Nanowires (ANW) and Catalysts Synthesis
2.4. Characterization
2.5. Catalytic Activity
3. Results and Discussion
3.1. Support and Catalysts Characterization
3.2. Catalytic Activity
3.2.1. Effect of Au Loading
3.2.2. Effect of the Solvent
3.2.3. Reusability and Operational Stability of the Catalyst
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferretti, F.; Ramadan, D.R.; Ragaini, F. Transition Metal Catalyzed Reductive Cyclization Reactions of Nitroarenes and Nitroalkenes. ChemCatChem 2019, 11, 4450–4488. [Google Scholar] [CrossRef]
- Song, J.; Huang, Z.-F.; Pan, L.; Li, K.; Zhang, X.; Wang, L.; Zou, J.-J. Review on selective hydrogenation of nitroarene by catalytic, photocatalytic and electrocatalytic reactions. Appl. Catal. B Environ. 2018, 227, 386–408. [Google Scholar] [CrossRef]
- Ley, S.V.; Baxendale, I.R. New tools and concepts for modern organic synthesis. Nat. Rev. Drug Discov. 2002, 1, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Vennerstrom, J.L. Book Review of the Art of Drug Synthesis. J. Med. Chem. 2008, 51, 1502. [Google Scholar] [CrossRef]
- Romanazzi, G.; Fiore, A.M.; Mali, M.; Rizzuti, A.; Leonelli, C.; Nacci, A.; Mastrorilli, P.; Dell’Anna, M.M. Polymer supported Nickel nanoparticles as recyclable catalyst for the reduction of nitroarenes to anilines in aqueous medium. Mol. Catal. 2018, 446, 31–38. [Google Scholar] [CrossRef]
- Kadam, H.K.; Tilve, S.G. Advancement in methodologies for reduction of nitroarenes. RSC Adv. 2015, 5, 83391–83407. [Google Scholar] [CrossRef]
- Patil, Y.S.R.D. Chemoselective Reduction of Nitroarenes to Aromatic Amines with Commercial Metallic Iron Powder in Water under Mild Reaction Conditions. Org. Chem. Curr. Res. 2015, 4, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, K.-I.; Miyamoto, Y.; Kawasaki, T.; Tanji, T.; Tai, Y.; Satsuma, A. Chemoselective Hydrogenation of Nitroaromatics by Supported Gold Catalysts: Mechanistic Reasons of Size- and Support-Dependent Activity and Selectivity. J. Phys. Chem. C 2009, 113, 17803–17810. [Google Scholar] [CrossRef]
- Tamura, M.; Yuasa, N.; Nakagawa, Y.; Tomishige, K. Selective hydrogenation of nitroarenes to aminoarenes using a MoOx-modified Ru/SiO2 catalyst under mild conditions. Chem. Commun. 2017, 53, 3377–3380. [Google Scholar] [CrossRef]
- Tian, H.; Zhou, J.; Li, Y.; Wang, Y.; Liu, L.; Ai, Y.; Hu, Z.-N.; Li, J.; Guo, R.; Liu, Z.; et al. Rh Catalyzed Selective Hydrogenation of Nitroarenes under Mild Conditions: Understanding the Functional Groups Attached to the Nanoparticles. ChemCatChem 2019, 11, 5543–5552. [Google Scholar] [CrossRef]
- Corma, A. Chemoselective Hydrogenation of Nitro Compounds with Supported Gold Catalysts. Science 2006, 313, 332–334. [Google Scholar] [CrossRef] [PubMed]
- Torres, C.; Campos, C.; Fierro, J.L.G.; Oportus, M.; Reyes, P. Nitrobenzene Hydrogenation on Au/TiO2 and Au/SiO2 Catalyst: Synthesis, Characterization and Catalytic Activity. Catal. Lett. 2013, 143, 763–771. [Google Scholar] [CrossRef]
- Campos, C.; Jofre, M.; Torres, C.; Pawelec, B.; Fierro, J.; Reyes, P. Chemoselective hydrogenation of o-, p- and m-chloronitrobenzene at ambient temperature on Au/Fe2O3 catalysts. Appl. Catal. A Gen. 2014, 482, 127–136. [Google Scholar] [CrossRef]
- Wang, X.; Cárdenas-Lizana, F.; Keane, M.A. Toward Sustainable Chemoselective Nitroarene Hydrogenation Using Supported Gold as Catalyst. ACS Sustain. Chem. Eng. 2014, 2, 2781–2789. [Google Scholar] [CrossRef]
- Serna, P.; Corma, A. Transforming Nano Metal Nonselective Particulates into Chemoselective Catalysts for Hydrogenation of Substituted Nitrobenzenes. ACS Catal. 2015, 5, 7114–7121. [Google Scholar] [CrossRef]
- Torres, C.C.; Alderete, J.B.; Pecchi, G.; Campos, C.H.; Reyes, P.; Pawelec, B.; Vaschetto, E.G.; Eimer, G.A. Heterogeneous hydrogenation of nitroaromatic compounds on gold catalysts: Influence of titanium substitution in MCM-41 mesoporous supports. Appl. Catal. A Gen. 2016, 517, 110–119. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Wang, H.; Shao, Y.; Liu, X.; Wang, Y.-Q.; Lewis, J.P.; Xiao, F.-S. Activity and Selectivity in Nitroarene Hydrogenation over Au Nanoparticles on the Edge/Corner of Anatase. ACS Catal. 2016, 6, 4110–4116. [Google Scholar] [CrossRef]
- Torres, C.C.; Jiménez, V.A.; Campos, C.H.; Alderete, J.B.; Dinamarca, R.; Bustamente, T.M.; Pawelec, B. Gold catalysts supported on TiO2-nanotubes for the selective hydrogenation of p-substituted nitrobenzenes. Mol. Catal. 2018, 447, 21–27. [Google Scholar] [CrossRef]
- Begum, R.; Rehan, R.; Farooqi, Z.H.; Butt, Z.; Ashraf, S. Physical chemistry of catalytic reduction of nitroarenes using various nanocatalytic systems: Past, present, and future. J. Nanopartic. Res. 2016, 18, 231. [Google Scholar] [CrossRef]
- Perez-Lloret, M.; Fraix, A.; Petralia, S.; Conoci, S.; Tafani, V.; Cutrone, G.; Vargas-Berenguel, A.; Gref, R.; Sortino, S. One-Step Photochemical Green Synthesis of Water-Dispersible Ag, Au, and Au@Ag Core–Shell Nanoparticles. Chem. A Eur. J. 2019, 25, 14638–14643. [Google Scholar] [CrossRef]
- Shin, H.-S.; Huh, S. Au/Au@Polythiophene Core/Shell Nanospheres for Heterogeneous Catalysis of Nitroarenes. ACS Appl. Mater. Interf. 2012, 4, 6324–6331. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Zheng, J.; Zhang, L.; Yi, Z. Core-Shell Au@SnO2 Nanostructures Supported on Na2Ti4O9 Nanobelts as a Highly Active and Deactivation-Resistant Catalyst toward Selective Nitroaromatics Reduction. Inorg. Chem. 2019, 58, 11164–11171. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Qiao, J.; Wang, J.; Wu, H.; Wang, Z.; Sun, W.; Sun, K. Multimetallic Core–Bishell Ni@Au@Pd nanoparticles with reduced graphene oxide as an efficient bifunctional electrocatalyst for oxygen reduction/evolution reactions. J. Alloys Compd. 2019, 811. [Google Scholar] [CrossRef]
- Cano, M.; Villuendas, P.; Benito, A.M.; Urriolabeitia, E.P.; Maser, W.K. Carbon nanotube-supported gold nanoparticles as efficient catalyst for the selective hydrogenation of nitroaromatic derivatives to anilines. Mater. Today Commun. 2015, 3, 104–113. [Google Scholar] [CrossRef] [Green Version]
- Glotov, A.; Stavitskaya, A.; Chudakov, Y.; Ivanov, E.; Huang, W.; Vinokurov, V.; Zolotukhina, A.; Maximov, A.; Karakhanov, E.; Lvov, Y. Mesoporous Metal Catalysts Templated on Clay Nanotubes. Bull. Chem. Soc. Jpn. 2019, 92, 61–69. [Google Scholar] [CrossRef]
- Jia, L.; Zhou, T.; Xu, J.; Li, F.; Xu, Z.; Zhang, B.; Guo, S.; Shen, X.; Zhang, W. AuPd Bimetallic Nanocrystals Embedded in Magnetic Halloysite Nanotubes: Facile Synthesis and Catalytic Reduction of Nitroaromatic Compounds. Nanomaterials 2017, 7, 333. [Google Scholar] [CrossRef] [Green Version]
- Leandro, S.R.; Fernandes, C.I.; Viana, A.S.; Mourato, A.C.; Vaz, P.D.; Nunes, C.D. Catalytic performance of bulk and colloidal Co/Al layered double hydroxide with Au nanoparticles in aerobic olefin oxidation. Appl. Catal. A Gen. 2019, 584. [Google Scholar] [CrossRef]
- Liu, W.; Sun, D.; Fu, J.; Yuan, R.; Li, Z. Assembly of evenly distributed Au nanoparticles on thiolated reduced graphene oxide as an active and robust catalyst for hydrogenation of 4-nitroarenes. RSC Adv. 2014, 4, 11003–11011. [Google Scholar] [CrossRef]
- Rocha, M.; Costa, P.; Sousa, C.A.; Pereira, C.; Rodriguez-Borges, J.E.; Freire, C. l-serine-functionalized montmorillonite decorated with Au nanoparticles: A new highly efficient catalyst for the reduction of 4-nitrophenol. J. Catal. 2018, 361, 143–155. [Google Scholar] [CrossRef]
- Hosseini, S.H.; Zohreh, N.; Alipour, S.; Busuioc, C.; Negrea, R. Gold nanoparticles stabilized on SBA-15 functionalized NNN-pincer ligand; highly effective catalyst for reduction of nitroarenes in aqueous medium. Catal. Commun. 2018, 108, 93–97. [Google Scholar] [CrossRef]
- Fan, H.-T.; Liu, X.-G.; Xing, X.-J.; Li, B.; Wang, K.; Chen, S.-T.; Wu, Z.; Qiu, D.-F. Ordered mesoporous silica cubic particles decorated with silver nanoparticles: A highly active and recyclable heterogeneous catalyst for the reduction of 4-nitrophenol. Dalton Trans. 2019, 48, 2692–2700. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Feng, X.; Huang, D.; Yang, G.; Astruc, D. Basic concepts and recent advances in nitrophenol reduction by gold- and other transition metal nanoparticles. Coord. Chem. Rev. 2015, 287, 114–136. [Google Scholar] [CrossRef]
- Qu, R.; Macino, M.; Iqbal, S.; Gao, X.; He, Q.; Hutchings, G.J.; Sankar, M. Supported Bimetallic AuPd Nanoparticles as a Catalyst for the Selective Hydrogenation of Nitroarenes. Nanomaterials 2018, 8, 690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, H.; Zhang, L.; Wang, Y.; Chen, S.; Wan, Y. Thermally reduced gold nanocatalysts prepared by the carbonization of ordered mesoporous carbon as a heterogeneous catalyst for the selective reduction of aromatic nitro compounds. J. Catal. 2016, 344, 313–324. [Google Scholar] [CrossRef]
- Lokteva Ekaterina, S.; Golubina Elena, V. Metal-support interactions in the design of heterogeneous catalysts for redox processes. Pure Appl. Chem. 2019, 91, 609. [Google Scholar] [CrossRef]
- Shi, H. Valorization of Biomass-derived Small Oxygenates: Kinetics, Mechanisms and Site Requirements of H2-involved Hydrogenation and Deoxygenation Pathways over Heterogeneous Catalysts. ChemCatChem 2019, 11, 1824–1877. [Google Scholar] [CrossRef]
- Dong, H.; Xie, R.; Yang, L.; Li, F. A hierarchical flower-like hollow alumina supported bimetallic AuPd nanoparticle catalyst for enhanced solvent-free ethylbenzene oxidation. Dalton Trans. 2018, 47, 7776–7786. [Google Scholar] [CrossRef]
- Chen, L.; Yan, J.; Tong, Z.; Yu, S.; Tang, J.; Ou, B.; Yue, L.; Tian, L. Nanofiber-like mesoporous alumina supported palladium nanoparticles as a highly active catalyst for base-free oxidation of benzyl alcohol. Microp. Mesop. Mater. 2018, 266, 126–131. [Google Scholar] [CrossRef]
- Nikoofar, K.; Shahedi, Y.; Chenarboo, F.J. Nano Alumina Catalytic Applications in Organic Transformations. Mini Rev. Org. Chem. 2019, 16, 102–110. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Tiwari, R.N.; Kim, K.S. Zero-dimensional, one-dimensional, two-dimensional and three-dimensional nanostructured materials for advanced electrochemical energy devices. Prog. Mater. Sci. 2012, 57, 724–803. [Google Scholar] [CrossRef]
- Lu, C.L.; Lv, J.G.; Xu, L.; Guo, X.F.; Hou, W.H.; Hu, Y.; Huang, H. Crystalline nanotubes of γ-AlOOH and γ-Al2O3: Hydrothermal synthesis, formation mechanism and catalytic performance. Nanotechnology 2009, 20, 215604. [Google Scholar] [CrossRef]
- Wang, X.; Perret, N.; Delgado, J.J.; Blanco, G.; Chen, X.; Olmos, C.M.; Bernal, S.; Keane, M.A. Reducible Support Effects in the Gas Phase Hydrogenation of p-Chloronitrobenzene over Gold. J. Phys. Chem. C 2013, 117, 994–1005. [Google Scholar] [CrossRef]
- Wang, X.; Perret, N.; Keane, M.A. The role of hydrogen partial pressure in the gas phase hydrogenation of p-chloronitrobenzene over alumina supported Au and Pd: A consideration of reaction thermodynamics and kinetics. Chem. Eng. J. 2012, 210, 103–113. [Google Scholar] [CrossRef]
- Yakushkin, S.S.; Nuzhdin, A.L.; Artiukha, E.A.; Plyusnin, P.E.; Bukhtiyarova, G.A.; Martyanov, O.N. In situ EPR study of chemoselective hydrogenation of nitroarenes on Au/Al2O3 catalyst. Mendeleev Commun. 2018, 28, 536–537. [Google Scholar] [CrossRef]
- Mali, A.C.; Deshmukh, D.G.; Joshi, D.R.; Lad, H.D.; I Patel, P.; Medhane, V.J.; Mathad, V.T. Facile approach for the synthesis of rivaroxaban using alternate synthon: Reaction, crystallization and isolation in single pot to achieve desired yield, quality and crystal form. Sustain. Chem. Process. 2015, 3, 5900. [Google Scholar] [CrossRef]
- Gardiner, J.; Nguyen, X.; Genet, C.; Horne, M.D.; Hornung, C.H.; Tsanaktsidis, J. Catalytic Static Mixers for the Continuous Flow Hydrogenation of a Key Intermediate of Linezolid (Zyvox). Org. Process. Res. Dev. 2018, 22, 1448–1452. [Google Scholar] [CrossRef]
- Bustamante, T.M.; Dinamarca, R.; Torres, C.C.; Pecchi, G.; Campos, C.H. Pd-Co catalysts prepared from palladium-doped cobalt titanate precursors for chemoselective hydrogenation of halonitroarenes. Mol. Catal. 2019. [Google Scholar] [CrossRef]
- Bharath, Y.; Alugubelli, G.R.; Sreenivasulu, R.; Rao, M.V.B. Design, synthesis of novel oxazolidino-amides/sulfonamides conjugates and their impact on antibacterial activity. Chem. Pap. 2018, 72, 457–468. [Google Scholar] [CrossRef]
- Xing, J.; Yang, L.; Li, H.; Li, Q.; Zhao, L.; Wang, X.; Zhang, Y.; Zhou, M.; Zhou, J.; Zhang, H. Identification of anthranilamide derivatives as potential factor Xa inhibitors: Drug design, synthesis and biological evaluation. Eur. J. Med. Chem. 2015, 95, 388–399. [Google Scholar] [CrossRef]
- Markgraf, J.H.; Stickney, C.A. A new synthesis of N-phenyl lactams. J. Heterocycl. Chem. 2000, 37, 109–110. [Google Scholar] [CrossRef]
- Campos, C.H.; Díaz, C.F.; Guzmán, J.L.; Alderete, J.B.; Torres, C.C.; Jiménez, V.A. PAMAM-Conjugated Alumina Nanotubes as Novel Noncytotoxic Nanocarriers with Enhanced Drug Loading and Releasing Performances. Macromol. Chem. Phys. 2016, 217, 1712–1722. [Google Scholar] [CrossRef]
- Dinamarca, R.B.; Espinoza-González, R.; Campos, C.H.; Pecchi, G. Magnetic Pt single and double core-shell structures for the catalytic selective hydrogenation of cinnmaladehyde. Pure Appl. Chem. 2019. [Google Scholar] [CrossRef] [Green Version]
- Campos, C.H.; Torres, C.; Fierro, J.L.; Reyes, P. Enantioselective hydrogenation of 1-phenyl-1,2-propanedione over Pt on immobilized cinchonidine on γ-Al2O3 catalysts. Appl. Catal. A Gen. 2013, 466, 198–207. [Google Scholar] [CrossRef]
- Liu, W.; Hiekel, K.; Hübner, R.; Sun, H.; Ferancova, A.; Sillanpää, M. Pt and Au bimetallic and monometallic nanostructured amperometric sensors for direct detection of hydrogen peroxide: Influences of bimetallic effect and silica support. Sens. Actuators B Chem. 2018, 255, 1325–1334. [Google Scholar] [CrossRef]
- Bogdanchikova, N.; Pestryakov, A.; Farías, M.; Diaz, J.; Avalos, M.; Navarrete, J. Formation of TEM- and XRD-undetectable gold clusters accompanying big gold particles on TiO2–SiO2 supports. Solid State Sci. 2008, 10, 908–914. [Google Scholar] [CrossRef]
- Sobczak, I.; Calvino-Casilda, V.; Wolski, L.; Siodla, T.; Martin-Aranda, R.; Ziolek, M. The role of gold dopant in AP-Nb/MCF and AP-MCF on the Knoevenagel condensation of ethyl cyanoacetate with benzaldehyde and 2,4-dichlorobenzaldehyde. Catal. Today 2019, 325, 81–88. [Google Scholar] [CrossRef]
- Tuzovskaya, I.; Bogdanchikova, N.; Simakov, A.; Gurin, V.; Pestryakov, A.; Avalos, M.; Farías, M. Structure and electronic states of gold species in mordenites. Chem. Phys. 2007, 338, 23–32. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, B.; Yue, Y.; Di, S.; Zhai, Y.; He, H.; Sheng, G.; Lai, H.; Zhu, Y.; Guo, L.; et al. Towards a greener approach for the preparation of highly active gold/carbon catalyst for the hydrochlorination of ethyne. J. Catal. 2018, 365, 153–162. [Google Scholar] [CrossRef]
- Wojcieszak, R.; Genet, M.J.; Eloy, P.; Ruiz, P.; Gaigneaux, E.M. Determination of the Size of Supported Pd Nanoparticles by X-ray Photoelectron Spectroscopy. Comparison with X-ray Diffraction, Transmission Electron Microscopy, and H2 Chemisorption Methods. J. Phys. Chem. C 2010, 114, 16677–16684. [Google Scholar] [CrossRef]
- Yang, X.; Yang, Z.; Tang, F.; Xu, J.; Zhang, M.; Choi, M.M.F. Structural and optical properties of penicillamine-protected gold nanocluster fractions separated by sequential size-selective fractionation. Beilstein J. Nanotechnol. 2019, 10, 955–966. [Google Scholar] [CrossRef]
- Cárdenas-Lizana, F.; Lamey, D.; Gómez-Quero, S.; Perret, N.; Kiwi-Minsker, L.; Keane, M.A. Selective three-phase hydrogenation of aromatic nitro-compounds over β-molybdenum nitride. Catal. Today 2011, 173, 53–61. [Google Scholar] [CrossRef]
- Xiong, H.; Pham, H.N.; Datye, A.K. Hydrothermally stable heterogeneous catalysts for conversion of biorenewables. Green Chem. 2014, 16, 4627–4643. [Google Scholar] [CrossRef]
- Sievers, C.; Noda, Y.; Qi, L.; Albuquerque, E.M.; Rioux, R.M.; Scott, S.L. Phenomena Affecting Catalytic Reactions at Solid–Liquid Interfaces. ACS Catal. 2016, 6, 8286–8307. [Google Scholar] [CrossRef]
- Furimsky, E. Hydroprocessing in Aqueous Phase. Ind. Eng. Chem. Res. 2013, 52, 17695–17713. [Google Scholar] [CrossRef]












| Material | Au Loading (%) | SBET (m2g−1) | ANW dpore (nm) | Au dTEM (nm) |
|---|---|---|---|---|
| ANW | - | 204 | 8.9 | - |
| ANW-NH2 | - | 154 | 8.5 | - |
| 0.25Au/ANW | 0.21 | 138 | 8.0 | 3.7 ± 1.8 |
| 0.50Au/ANW | 0.42 | 126 | 8.1 | 4.0 ± 1.1 |
| 1.00Au/ANW | 0.87 | 115 | 7.9 | 4.6 ± 2.6 |
| x | Al 2p (eV) | Au 4f7/2 (eV) | O 1s (eV) | Au/Al at | |
|---|---|---|---|---|---|
| Au0 | Auδ+ | ||||
| 0.25 | 74.5 | 83.6 (91) | 84.8 (9) | 531.4 | 0.022 |
| 0.50 | 74.5 | 83.8 (84) | 85.0 (16) | 531.3 | 0.031 |
| 1.00 | 74.4 | 84.0 (78) | 85.1 (22) | 531.3 | 0.009 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shanmugaraj, K.; Bustamante, T.M.; Campos, C.H.; Torres, C.C. Liquid Phase Hydrogenation of Pharmaceutical Interest Nitroarenes over Gold-Supported Alumina Nanowires Catalysts. Materials 2020, 13, 925. https://doi.org/10.3390/ma13040925
Shanmugaraj K, Bustamante TM, Campos CH, Torres CC. Liquid Phase Hydrogenation of Pharmaceutical Interest Nitroarenes over Gold-Supported Alumina Nanowires Catalysts. Materials. 2020; 13(4):925. https://doi.org/10.3390/ma13040925
Chicago/Turabian StyleShanmugaraj, Krishnamoorthy, Tatiana M. Bustamante, Cristian H. Campos, and Cecilia C. Torres. 2020. "Liquid Phase Hydrogenation of Pharmaceutical Interest Nitroarenes over Gold-Supported Alumina Nanowires Catalysts" Materials 13, no. 4: 925. https://doi.org/10.3390/ma13040925