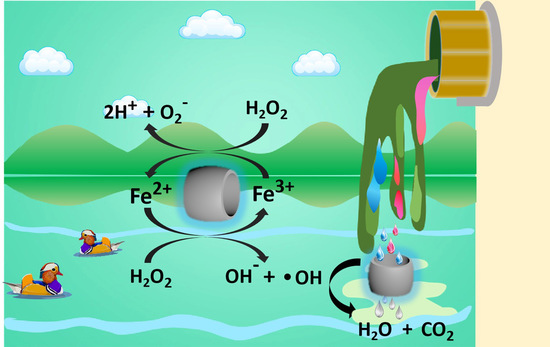
A Fenton-Like Nanocatalyst Based on Easily Separated Magnetic Nanorings for Oxidation and Degradation of Dye Pollutant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Synthesis of Fe3O4-MNRs
2.3. Characterization
2.4. Free Radical (•OH) Generation and Detection
2.5. Catalytic Activity Measurements
2.6. The Degradation Mechanism
2.7. Statistical Analysis
3. Results
3.1. Morphology
3.2. Size Distributions
3.3. Magnetization
3.4. X-ray Diffraction
3.5. X-ray Photoelectron Spectroscopy
3.6. Hydroxyl Radicals (•OH) Generation and Monitoring
3.7. Dye Degradation through a Catalytic Fenton-Like Reaction
3.8. Dye Degradation Study
3.9. Regeneration of the Catalyst
3.10. The Degradation Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fang, Y.Y.; Huang, Q.Z.; Liu, P.Y.; Shi, J.F.; Xu, G. A facile dip-coating method for the preparation of separable MoS2 sponges and their high-efficient adsorption behaviors of Rhodamine, B. Inorg. Chem. Front. 2018, 5, 827–834. [Google Scholar] [CrossRef]
- Zhu, Q.; Liu, N.; Zhang, N.; Song, Y.Y.; Stanislaus, M.S.; Zhao, C.Y.; Yang, Y.N. Efficient Photocatalytic Removal of RhB, MO and MB Dyes by Op timized Ni/NiO/TiO2 Composite Thin Films under Solar Light Irradiation. J. Environ. Chem. Eng. 2018, 6, 2724–2732. [Google Scholar] [CrossRef]
- Tu, T.H.; Cam, P.T.N.; Huy, L.V.T.; Phong, M.T.; Nam, H.M.; Hieu, N.H. Synthesis and application of graphene oxide aerogel as an adsorbent for removal of dyes from water. Mater. Lett. 2018, 238, 134–137. [Google Scholar] [CrossRef]
- Crini, G. Non-conventional low-cost adsorbents for dye removal: A review. Bioresour. Technol. 2006, 97, 1061–1085. [Google Scholar] [CrossRef]
- Kurtan, U.; Amir, M.; Baykal, A. Fe3O4@Nico-Ag magnetically recyclable nanocatalyst for azo dyes reduction. Appl. Surf. Sci. 2016, 363, 66–73. [Google Scholar] [CrossRef]
- Buscio, V.; Garcia-Jimenez, M.; Vilaseca, M.; Lopez-Grimau, V.; Crespi, M.; Gutierrez-Bouzan, C. Reuse of Textile Dyeing Effluents Treated with Coupled Nanofiltration and Electrochemical Processes. Materials 2016, 9, 490. [Google Scholar] [CrossRef]
- Vyrides, I.; Bonakdarpour, B.; Stuckey, D.C. Salinity effects on biodegradation of Reactive Black 5 for one stage and two stages sequential anaerobic aerobic biological processes employing different anaerobic sludge. Int. Biodeterior. Biodegrad. 2014, 95, 294–300. [Google Scholar] [CrossRef]
- Lotito, A.M.; De Sanctis, M.; Di Iaconi, C.; Bergna, G. Textile wastewater treatment: Aerobic granular sludge vs activated sludge systems. Water Res. 2014, 54, 337–346. [Google Scholar] [CrossRef]
- Lau, Y.Y.; Wong, Y.S.; Teng, T.T.; Morad, N.; Rafatullah, M.; Ong, S.-A. Coagulation-flocculation of azo dye Acid Orange 7 with green refined laterite soil. Chem. Eng. J. 2014, 246, 383–390. [Google Scholar] [CrossRef]
- Kumar, S.; Khanchandani, S.; Thirumal, M.; Ganguli, A.K. Achieving Enhanced Visible-Light-Driven Photocatalysis Using Type-II NaNbO3/CdS Core/Shell Heterostructures. ACS Appl. Mater. Interfaces 2014, 6, 13221–13233. [Google Scholar] [CrossRef]
- Zhou, L.C.; Shao, Y.M.; Liu, J.R.; Ye, Z.F.; Zhang, H.; Ma, J.; Jia, Y.; Gao, W.J.; Li, Y.F. Preparation and Characterization of Magnetic Porous Carbon Microspheres for Removal of Methylene Blue by a Heterogeneous Fenton Reaction. ACS Appl. Mater. Interfaces 2014, 6, 7275–7285. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.M.; Chen, Z.; Cao, C.Y.; Jiang, L.; Song, W.G. A yolk-shell structured Fe2O3@mesoporous SiO2 nanoreactor for enhanced activity as a Fenton catalyst in total oxidation of dyes. Chem. Commun. 2013, 49, 2332–2334. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.H.; Mitch, W.A. The Effect of Ozonation and Biological Activated Carbon Treatment of Wastewater Effluents on Formation of Nnitrosamines and Halogenated Disinfection Byproducts. Environ. Sci. Technol. 2017, 51, 2329–2338. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Bai, Z.Y. Fe-based catalysts for heterogeneous catalytic ozonation of emerging contaminants in water and wastewater. Chem. Eng. J. 2017, 312, 79–98. [Google Scholar] [CrossRef]
- Jiménez, S.; Andreozzi, M.; Micó, M.M.; Álvarez, M.G.; Contreras, S. Produced water treatment by advanced oxidation processes. Sci. Total Environ. 2019, 666, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.M.; Zu, L.H.; Jiang, Y.; Shi, D.L.; Cai, X.M.; Ni, Y.H.; Lin, S.J.; Qin, Y. A titanium-based photo-Fenton bifunctional catalyst of mp-MXene/TiO2−x nanodots for dramatic enhancement of catalytic efficiency in advanced oxidation processes. Chem. Commun. 2018, 54, 11622–11625. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.Y.; Hu, X.Y.; Wang, C.H.; Tai, Y.F. Water-dispersible and recyclable magnetic TiO2/graphene nanocomposites in wastewater treatment. Mater. Lett. 2018, 231, 80–83. [Google Scholar] [CrossRef]
- Deng, F.; Zhong, F.; Zhao, L.N.; Luo, X.B.; Luo, S.L.; Dionysiou, D.D. One-step in situ hydrothermal fabrication of octahedral CdS/SnIn4S8 nano-heterojunction for highly efficient photocatalytic treatment of nitrophenol and real pharmaceutical wastewater. J. Hazard. Mater. 2017, 340, 85–95. [Google Scholar] [CrossRef]
- Ji, B.; Zhang, J.X.; Zhang, C.; Li, N.; Zhao, T.T.; Chen, F.; Hu, L.H.; Zhang, S.D.; Wang, Z.Y. Vertically-aligned ZnO@ZnS nanorod chip with improved photocatalytic activity for antibiotics degradation. ACS Appl. Nano Mater. 2018, 1, 793–799. [Google Scholar] [CrossRef]
- Kim, H.; Watthanaphanit, A.; Saito, N.; Saito, N. Simple Solution Plasma Synthesis of Hierarchical Nanoporous MnO2 for Organic Dye Removal. ACS Sustain. Chem. Eng. 2017, 5, 5842–5851. [Google Scholar] [CrossRef]
- Sun, B.F.; Li, H.L.; Li, X.Y.; Liu, X.W.; Zhang, C.H.; Xu, H.Y.; Zhao, X.S. Degradation of Organic Dyes over Fenton-Like Cu2O−Cu/C Catalysts. Ind. Eng. Chem. Res. 2018, 57, 14011–14021. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.H.; Han, Q.F.; Yao, X.X.; Wang, X. Formation of WO3 nanotube-based bundles directed by NaHSO4 and its application in water treatment. J. Mater. Chem. A 2013, 1, 1246–1253. [Google Scholar] [CrossRef]
- Nguyen, X.S.; Zhang, G.K.; Yang, X.F. Mesocrystalline Zn-Doped Fe3O4 Hollow Submicrospheres: Formation Mechanism and Enhanced Photo-Fenton Catalytic Performance. ACS Appl. Mater. Interfaces 2017, 9, 8900–8909. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, G.; Madras, G. Remarkable enhancement of Fenton degradation at a wide pH range promoted by thioglycolic acid. Chem. Commun. 2017, 53, 1136–1139. [Google Scholar] [CrossRef] [PubMed]
- Saleh, R.; Taufic, A. Degradation of methylene blue and congo-red dyes using Fenton, photo-Fenton, sono-Fenton, and sonophoto-Fenton methods in the presence of iron (II,III) oxide/zinc oxide/graphene (Fe3O4/ZnO/graphene) composites. Sep. Purif. Technol. 2019, 210, 563–573. [Google Scholar] [CrossRef]
- Hartmann, M.; Kullmanna, S.; Keller, H. Wastewater treatment with heterogeneous Fenton-type catalysts based on porous materials. J. Mater. Chem. 2010, 20, 9002–9017. [Google Scholar] [CrossRef]
- Xu, H.Y.; Wang, Y.; Shi, T.N.; Zhao, H.; Tan, Q.; Zhao, B.C.; He, X.L.; Qi, S.Y. Heterogeneous Fenton-like discoloration of methyl orange using Fe3O4/MWCNTs as catalyst: Kinetics and Fenton-like mechanism. Front. Mater. Sci. 2018, 12, 34–44. [Google Scholar] [CrossRef]
- Lopez-Tejedor, D.; Benavente, R.; Palomo, J.M. Iron nanostructured catalysts: Design and applications. Catal. Sci. Technol. 2018, 8, 1754–1776. [Google Scholar] [CrossRef]
- Jia, C.J.; Sun, L.D.; Luo, F.; Han, X.D.; Heyderman, L.; Yan, Z.; Yan, C.H.; Zheng, K.; Zhang, Z.; Takano, M.; et al. Large-Scale Synthesis of Single-Crystalline Iron Oxide Magnetic Nanorings. J. Am. Chem. Soc. 2008, 130, 16968–16977. [Google Scholar] [CrossRef] [Green Version]
- Zhou, R.; Shen, N.F.; Zhao, J.; Su, Y.; Ren, H.J. Glutathione-coated Fe3O4 nanoparticles with enhanced Fenton-like activity at neutral pH for degrading 2,4-dichlorophenol. J. Mater. Chem. A 2018, 6, 1275–1283. [Google Scholar] [CrossRef]
- He, Y.; Jang, D.B.; Jiang, D.Y.; Chen, J.; Zhang, Y.X. Evaluation of MnO2-templated iron oxide-coated diatomites for their catalytic performance in heterogeneous photo Fenton-like system. J. Hazard. Mater. 2018, 344, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Cui, K.X.; Yan, B.; Xie, Y.J.; Qian, H.; Wang, X.G.; Huang, Q.X.; He, Y.H.; Jin, S.M.; Zeng, H.B. Regenerable Urchin-like Fe3O4@PDA-Ag Hollow Microspheres as Catalyst and Adsorbent for Enhanced Removal of Organic Dyes. J. Hazard. Mater. 2018, 350, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Vilela, C.; Moreirinha, C.; Almeida, A.; Silvestre, A.J.D.; Freire, C.S.R. Zwitterionic Nanocellulose-Based Membranes for Organic Dye Removal. Materials 2019, 12, 1404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouyang, J.; Zhao, Z.; Suib, S.L.; Yang, H.M. Degradation of Congo Red dye by a Fe2O3@CeO2-ZrO2/Palygorskite composite catalyst: Synergetic effects of Fe2O3. J. Colloid Interf. Sci. 2019, 539, 135–145. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, B.B.; Wang, Q.; Xing, S.T. Facile synthesis of α-FeOOH/γ-Fe2O3 by a pH gradient method and the role of γ-Fe2O3 in H2O2 activation under visible light irradiation. Chem. Eng. J. 2018, 354, 75–84. [Google Scholar] [CrossRef]













© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Li, J.; Shi, W.; Bao, J.; Yang, X. A Fenton-Like Nanocatalyst Based on Easily Separated Magnetic Nanorings for Oxidation and Degradation of Dye Pollutant. Materials 2020, 13, 332. https://doi.org/10.3390/ma13020332
Li X, Li J, Shi W, Bao J, Yang X. A Fenton-Like Nanocatalyst Based on Easily Separated Magnetic Nanorings for Oxidation and Degradation of Dye Pollutant. Materials. 2020; 13(2):332. https://doi.org/10.3390/ma13020332
Chicago/Turabian StyleLi, Xiaonan, Jinghua Li, Weilu Shi, Jianfeng Bao, and Xianyuan Yang. 2020. "A Fenton-Like Nanocatalyst Based on Easily Separated Magnetic Nanorings for Oxidation and Degradation of Dye Pollutant" Materials 13, no. 2: 332. https://doi.org/10.3390/ma13020332