Exploring Avoided Environmental Impacts as Well as Energy and Resource Recovery from Microbial Desalination Cell Treatment of Brine
Abstract
:1. Introduction
1.1. Brine Production
1.2. Microbial Desalination Cells (MDCs): An Emerging Technology
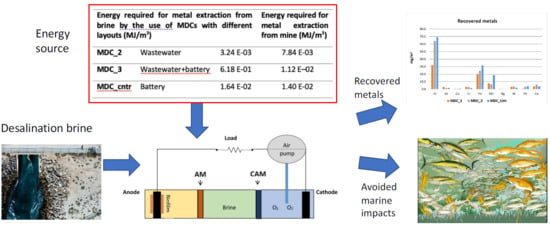
1.3. Goals of the Paper
2. Materials and Methods
2.1. MDCs’ Setup
2.2. Liquid Media
2.3. DMCs’ Operational Conditions
2.4. Chemical Analyses
2.5. MDCs’ Energy Inputs
2.6. Life Cycle Analysis (LCA)
3. Results and Discussion
3.1. Polarization Behavior and Energy Outputs
3.2. Microbiological Analyses
3.3. Chemical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xevgenos, D.; Moustakas, K.; Malamis, D.; Loizidou, M. An overview on desalination & sustainability: Renewable energy driven desalination and brine management. Desalination Water Treat. 2016, 57, 2304–2314. [Google Scholar] [CrossRef]
- Jones, E.; Qadir, M.; van Vliet, M.T.; Smakhtin, V.; Kang, S.M. The state of desalination and brine production: A global outlook. Sci. Total Environ. 2019, 657, 1343–1356. [Google Scholar] [CrossRef]
- Pistocchi, A.; Bleninger, T.; Dorati, C. Screening the hurdles to sea disposal of desalination brine around the Mediterranean. Desalination 2020, 491, 114570. [Google Scholar] [CrossRef]
- Frank, H.; Fussmann, K.E.; Rahav, E.; Zeev, E.B. Chronic effects of brine discharge from large-scale seawater reverse osmosis desalination facilities on benthic bacteria. Water Res. 2019, 151, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Giwa, A.; Dufour, V.; Al Marzooqi, F.; Al Kaabi, M.; Hasan, S.W. Brine management methods: Recent innovations and current status. Desalination 2017, 407, 1–23. [Google Scholar] [CrossRef]
- Panagopoulos, A.; Haralambous, K.J. Environmental impacts of desalination and brine treatment—Challenges and mitigation measures. Mar. Pollut. Bull. 2020, 161, 111773. [Google Scholar] [CrossRef]
- Garrote-Moreno, A.; Fernández-Torquemada, Y.; Sánchez-Lizaso, J.L. Salinity fluctuation of the brine discharge affects growth and survival of the seagrass Cymodocea nodosa. Mar. Pollut. Bull. 2014, 81, 61–68. [Google Scholar] [CrossRef]
- Panagopoulos, A.; Haralambous, K.; Loizidou, M. Desalination brine disposal methods and treatment technologies A review. Sci. Total Environ. 2019, 693, 133545. [Google Scholar] [CrossRef]
- Morillo, J.; Usero, J.; Rosado, D.; El Bakouri, H.; Riaza, A.; Bernaola, F.J. Comparative study of brine management technologies for desalination plants. Desalination 2014, 336, 32–49. [Google Scholar] [CrossRef]
- Almasri, D.; Khaled, A.; Mahmoud, K.A.; Abdel-Wahab, A. Two-stage sulfate removal from reject brine in inland desalination with zero-liquid discharge. Desalination 2015, 362, 52–58. [Google Scholar] [CrossRef]
- Tsai, J.H.; Macedonio, F.; Drioli, E.; Giorno, L.; Chou, C.Y.; Hu, F.C.; Li, C.; Chuang, C.J.; Tung, K.L. Membrane-based zero liquid discharge: Myth or reality? J. Taiwan Inst. Chem. E 2017, 80, 192–202. [Google Scholar] [CrossRef]
- Jafary, T.; Daud, W.R.W.; Aljlil, S.A.; Ismail, A.F.; Al-Mamun, A.; Baawain, M.S.; Ghasemi, M. Simultaneous organics, sulphate and salt removal in a microbial desalination cell with an insight into microbial communities. Desalination 2018, 445, 204–212. [Google Scholar] [CrossRef]
- Al-Mamun, A.; Ahmad, W.; Baawain, M.S.; Khadem, M.; Dhar, B.R. A review of microbial desalination cell technology: Configurations, optimization and applications. J. Clean Prod. 2018, 183, 458–480. [Google Scholar] [CrossRef]
- Nastro, R.A. Microbial fuel cells in waste treatment: Recent advances. Int. J. Perform. Eng. 2014, 10, 367–376. [Google Scholar]
- Santoro, C.; Arbizzani, C.; Erable, B.; Ieropoulos, I. Microbial fuel cells: From fundamentals to applications. A review. J. Power Sources 2017, 356, 225244. [Google Scholar] [CrossRef]
- Gambino, E.; Chandrasekhar, K.; Nastro, R.A. SMFC as a tool for the removal of hydrocarbons and metals in the marine environment: A concise research update. Environ. Sci. Pollut. Res. 2021, 28, 30436–30451. [Google Scholar] [CrossRef]
- Falcucci, G.; Nastro, R.A.; Minutillo, M.; De Santis, E.; Erme, G.; Ulgiati, S.; Jannelli, E. Microbial fuel cells fed by solid organic waste: A preliminar experimental study, EFC 2013. In Proceedings of the 5th European Fuel Cell Piero Lunghi Conference 2013, 139–140 5th European Fuel Cell Piero Lunghi Conference and Exhibition, EFC 2013, Rome, Italy, 11–13 December 2013. Code 110891. [Google Scholar]
- Florio, C.; Nastro, R.A.; Flagiello, F.; Minutillo, M.; Pirozzi, D.; Pasquale, V.; Ausiello, A.; Toscano, G.; Jannelli, E.; Dumontet, S. Biohydrogen production from Solid Phase-Microbial Fuel Cell spent substrate: A preliminary study. J. Clean Prod. 2019, 227, 506–511. [Google Scholar] [CrossRef]
- Endreny, T.; Avignone-Rossa, C.; Nastro, R.A. Generating electricity with urban green infrastructure microbial fuel cells. J. Clean Prod. 2020, 263, 121337. [Google Scholar] [CrossRef]
- Jegathambal, P.; Nisha, R.R.; Parameswari, K.; Subathra, M.S.P. Desalination and removal off organic pollutants using electrobiochemical reactor. Appl. Water Sci. 2019, 9, 113. [Google Scholar] [CrossRef] [Green Version]
- Santoro, C.; Abad, F.B.; Serov, A.; Kodali, M.; Howe, K.J.; Soavi, F.; Atanassov, P. Supercapacitive microbial desalination cells: New class of power generating devices for reduction of salinity content. Appl. Energy 2017, 208, 25–36. [Google Scholar] [CrossRef]
- Rahman, S.; Jafary, T.; Al-Mamun, A.; Baawain, M.S.; Choudhury, M.R.; Alhaimali, H.; Siddiqi, S.A.; Dhar, B.R.; Sana, A.; Lam, S.S.; et al. Towards upscaling microbial desalination cell technology: A comprehensive review on current challenges and future prospects. J. Clean Prod. 2021, 288, 125597. [Google Scholar] [CrossRef]
- Carmalin, S.A.; Bhalambaal, V.M.; Lima, E.C.; Thirunavoukkarasu, M. Microbial desalination cell technology: Contribution to sustainable wastewater treatment process, current status and future applications. J. Environ. Chem. Eng. 2016, 4, 3468–3478. [Google Scholar] [CrossRef]
- Malakootian, M.; Mahdizadeh, H.; Nasiri, A.; Mirzaienia, F.; Hajhoseini, M.; Amirmahani, N. Investigation of the efficiency of microbial desalination cell in removal of arsenic from aqueous solutions. Desalination 2018, 438, 19–23. [Google Scholar] [CrossRef]
- Forrestal, C.; Xu, P.; Ren, Z. Sustainable desalination using a microbial capacitive desalination cell. Energy Environ. Sci. 2012, 5, 7161. [Google Scholar] [CrossRef]
- Logan, B.; Cheng, S.; Watson, V.; Estadt, G. Graphite Fiber Brush Anodes for Increased Power Production in Air-Cathode Microbial Fuel Cells. Environ. Sci. Technol. 2007, 41, 3341–3346. [Google Scholar] [CrossRef]
- Guinée, J.B.; Lindeijer, E. Handbook on Life Cycle Assessment: Operational Guide to the ISO Standards; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Consoli, F.; Allen, D.; Boustead, I.; de Oude, N.; Fava, J.; Franklin, R.; Jensen, A.A.; Parrish, R.; Perriman, R.; Postlethwaite, D.; et al. Guidelines for Life-Cycle Assessment: A “Code of Practice”. In Proceedings of the SETAC Workshop, Sesimbra, Portugal, 31 March–3April 1993. [Google Scholar]
- International Organization for Standardization, Standard ISO, 14040 Environmental Management—Life Cycle Assessment—Principles and Framework. 2006. Available online: https://www.iso.org/standard/37456.html (accessed on 15 April 2021).
- International Organization for Standardization, Standard ISO, 14044 Environmental Management—Life Cycle Assessment—Requirements and Guidelines. Available online: https://www.iso.org/standard/38498.html (accessed on 15 April 2021).
- Corcelli, F.; Ripa, M.; Leccisi, E.; Cigolotti, V.; Fiandra, V.; Graditi, G.; Sannino, L.; Tammaro, M.; Ulgiati, S. Sustainable urban electricity supply chain–Indicators of material recovery and energy savings from crystalline silicon photovoltaic panels end-of-life. Ecol. Indic. 2018, 94, 37–51. [Google Scholar] [CrossRef] [Green Version]
- Buonocore, E.; Mellino, S.; De Angelis, G.; Liu, G.; Ulgiati, S. Life cycle assessment indicators of urban wastewater and sewage sludge treatment. Ecol. Indic. 2018, 94, 13–23. [Google Scholar] [CrossRef] [Green Version]
- Goedkoop, M.; Heijungs, R.; Huijbregts, M.; De Schryver, A.; Struijs, J.; Van Zelm, R. ReCiPe 2008. In A Life Cycle Impact Assessment Method Which Comprises Harmonized Category Indicators at the Midpoint and the Endpoint Level, 1st ed.; Version 1.08; Ministry of Housing Spatial Planning and the Environment: The Hague, The Netherlands, 2009; Volume 6, pp. 1–26. [Google Scholar]
- The Ecoinvent Database. Available online: https://www.ecoinvent.org/references/references.html (accessed on 15 April 2021).
- U.S. Geological Survey. Mineral Commodity Summaries 2020: U.S. Geological Survey; U.S. Geological Survey: Reston, VA, USA, 2020; ISBN 978-1-4113-4362-7. [Google Scholar] [CrossRef] [Green Version]
- Nuss, P.; Eckelman, M.J. Life cycle assessment of metals: A scientific synthesis. PLoS ONE 2014, 9, e101298. [Google Scholar] [CrossRef]
- Loganathan, P.; Naidu, G.; Vigneswaran, S. Mining valuable minerals from seawater: A critical review. Environ. Sci. Water Res. Technol. 2017, 3, 37–53. [Google Scholar] [CrossRef] [Green Version]





| Taxa | MDC_2 | MDC_3 |
|---|---|---|
| Bacillales_Incertae Sedis XII | 0 | 1.90 |
| Bifidobacteriaceae | 0.16 | 1.38 |
| Clostridiaceae 1 | 1.48 | 5.41 |
| Clostridiales_Incertae Sedis XI | 0.16 | 1.35 |
| Coriobacteriaceae | 0.00 | 1.55 |
| Incertae Sedis XI | 0.16 | 1.35 |
| Moraxellaceae | 30.87 | 53.72 |
| Parachlamydiaceae | 0 | 2.53 |
| Peptostreptococcaceae | 0.16 | 2.43 |
| Pseudomonadaceae | 52.55 | 1.85 |
| Unclassified_Acidimicrobiales | 0 | 1.07 |
| Unclassified_Actinomycetales | 0 | 1.73 |
| Unclassified_Alphaproteobacteria | 2.46 | 0.48 |
| Unclassified_Gammaproteobacteria | 7.06 | 3.02 |
| Xanthomonadaceae | 0 | 1.24 |
| Others < 1% | 4.93 | 18.99 |
| Parameters | Units of Measure | Brine | MDC_2_ | MDC_3_ | MDC_cntr_ |
|---|---|---|---|---|---|
| pH | 6.7 ± 0.2 | 6.6 ± 0.2 | 6.8 ± 0.2 | 6.8 ± 0.2 | |
| Cl- | mg/L | 228718 | 76771 | 80674 | 89636 |
| Br- | mg/L | 176 | 74.3 | 83 | 86.3 |
| SO4−2 | mg/L | 3937 | 1724 | 1521 | 1700 |
| Na+ | mg/L | 149934 | 63681 | 55412 | 64921 |
| K+ | mg/L | 4120 | 188 | 108 | 122 |
| Ca2+ | mg/L | 1853 | 1037 | 852 | 886 |
| Mg2+ | mg/L | 191 | 165 | 100 | 96.7 |
| Method: ReCiPe Midpoint (H) | Metals | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metric | Unit | Al | Sb | Co | Cr | Fe | Mn | Hg | Ni | Pb | Cu | Total Impact |
| Mid-point Human toxicity potential (HTP) | kg 1,4-DB eq/mg of recovered metal | 1.22 × 10−3 | 1.12 × 10−4 | 1.31 × 10−6 | 3.10 × 10−5 | 2.03 × 10−4 | 5.45 × 10−6 | 3.47 × 10−2 | 4.33 × 10−5 | 7.95 × 10−5 | 1.60 × 10−3 | 3.80 × 10−2 |
| Mid-point Marine eco-toxicity potential (ETP) | kg 1,4-DB eq/mg of recovered metal | 3.69 × 10−6 | 3.32 × 10−7 | 1.56 × 10−8 | 2.89 × 10−7 | 5.04 × 10−7 | 1.12 × 10−7 | 3.98 × 10−5 | 3.91 × 10−6 | 8.52 × 10−8 | 1.74 × 10−5 | 6.61 × 10−5 |
| Method: ReCiPe Endpoint (H) | Metals | |||||||||||
| Metric | Unit | Al | Sb | Co | Cr | Fe | Mn | Hg | Ni | Pb | Cu | Total Impact |
| End-point Human toxicity (HTP) | DALY/mg of recovered metal | 8.50 × 10−10 | 7.82 × 10−11 | 9.14 × 10−13 | 2.17 × 10−11 | 1.42 × 10−10 | 3.82 × 10−12 | 2.43 × 10−8 | 3.04 × 10−11 | 5.57 × 10−11 | 1.12 × 10−9 | 2.67 × 10−8 |
| End-point Marine ecotoxicity (ETP) | Species.yr/mg of recovered metal | 6.50 × 10−16 | 5.85 × 10−17 | 2.75 × 10−18 | 5.08 × 10−17 | 8.86 × 10−17 | 1.97 × 10−17 | 6.98 × 10−15 | 6.90 × 10−11 | 1.50 × 10−17 | 3.06 × 10−15 | 1.16 × 10−14 |
| Al | Sb | Co | Cr | Fe | Mn | Hg | Ni | Pb | Cu | Total Energy | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.384 | 0.008 | 0.002 | 0.016 | 0.147 | 0.038 | 0.0005 | 0.020 | 0.019 | 0.035 | n.a. |
| 0.413 | 0.009 | n.a. | 0.003 | 0.008 | 0.188 | 0.113 | 0.0004 | 0.012 | 0.022 | n.a. |
| 0.192 | 0.017 | 0.002 | 0.018 | 0.117 | 0.045 | 0.002 | 0.018 | 0.003 | 0.021 | n.a. |
| 0.024 | 0.117 | n.a. | 0.002 | 0.0002 | 0.012 | n.a. | n.a. | 0.008 | 0.024 | n.a. |
| 9.2 × 10−3 | 9.7 × 10−4 | n.a. | 3.2 × 10−5 | 2.9 × 10−5 | 4.7 × 10−4 | n.a. | n.a. | 1.5 × 10−4 | 8.5 × 10−4 | 1.12 × 10−2 |
| 9.93 × 10−3 | 1.16 × 10−3 | n.a. | 7.74 × 10−6 | 1.66 × 10−6 | 2.26 × 10−3 | n.a. | n.a. | 9.84 × 10−5 | 5.51 × 10−4 | 1.40 × 10−2 |
| 4.61 × 10−3 | 2.07 × 10−3 | n.a. | 3.64 × 10−5 | 2.35 × 10−5 | 5.43 × 10−4 | n.a. | n.a. | 2.90 × 10−5 | 5.23 × 10−4 | 7.84 × 10−3 |
| Desalination Process | Mining Practices | Source of Energy (Desalination) | |
|---|---|---|---|
| MDC_2 | 3.24 × 10−3 | 7.84 × 10−3 | Wastewater |
| MDC_3 | 6.18 × 10−1 | 1.12 × 10−2 | Wastewater + battery |
| MDC_cntr | 1.64 × 10−2 | 1.40 × 10−2 | Battery |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nastro, R.A.; Leccisi, E.; Toscanesi, M.; Liu, G.; Trifuoggi, M.; Ulgiati, S. Exploring Avoided Environmental Impacts as Well as Energy and Resource Recovery from Microbial Desalination Cell Treatment of Brine. Energies 2021, 14, 4453. https://doi.org/10.3390/en14154453
Nastro RA, Leccisi E, Toscanesi M, Liu G, Trifuoggi M, Ulgiati S. Exploring Avoided Environmental Impacts as Well as Energy and Resource Recovery from Microbial Desalination Cell Treatment of Brine. Energies. 2021; 14(15):4453. https://doi.org/10.3390/en14154453
Chicago/Turabian StyleNastro, Rosa Anna, Enrica Leccisi, Maria Toscanesi, Gengyuan Liu, Marco Trifuoggi, and Sergio Ulgiati. 2021. "Exploring Avoided Environmental Impacts as Well as Energy and Resource Recovery from Microbial Desalination Cell Treatment of Brine" Energies 14, no. 15: 4453. https://doi.org/10.3390/en14154453