COVID-19 and Breast Cancer: Analysis of Surgical Management of a Large Referral Center during the 2020–2021 Pandemic Period
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Study Population
2.2. Statistical Analysis
2.3. Ethical Approval
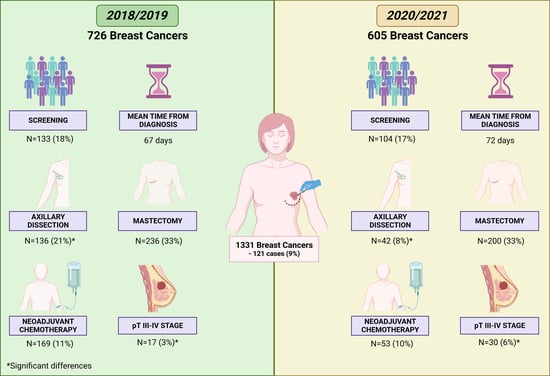
3. Results
3.1. Clinicopathological Features of the Entire Study Population
3.2. Comparison of Clinicopathological Features between the Pre-Pandemic and Pandemic Periods
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Coronavirus Disease (COVID-19)—Events as They Happen. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen (accessed on 25 October 2022).
- Shereen, M.A.; Khan, S.; Kazmi, A.; Bashir, N.; Siddique, R. COVID-19 infection: Origin, transmission, and characteristics of human coronaviruses. J. Adv. Res. 2020, 24, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Guzzetta, G.; Riccardo, F.; Marziano, V.; Poletti, P.; Trentini, F.; Bella, A.; COVID-19 Working Group. Impact of a Nationwide Lockdown on SARS-CoV-2 Transmissibility, Italy. Emerg. Infect. Dis. 2021, 27, 267–270. [Google Scholar] [CrossRef]
- Porto, E.D.; Naticchioni, P.; Scrutinio, V. Lockdown, essential sectors, and COVID-19: Lessons from Italy. J. Health Econ. 2022, 81, 102572. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Pesenti, A.; Cecconi, M. Critical Care Utilization for the COVID-19 Outbreak in Lombardy, Italy: Early Experience and Forecast During an Emergency Response. JAMA 2020, 323, 1545–1546. [Google Scholar] [CrossRef] [PubMed]
- Puci, M.V.; Nosari, G.; Loi, F.; Puci, G.V.; Montomoli, C.; Ferraro, O.E. Risk Perception and Worries among Health Care Workers in the COVID-19 Pandemic: Findings from an Italian Survey. Healthcare 2020, 8, 535. [Google Scholar] [CrossRef]
- Gorini, A.; Fiabane, E.; Sommaruga, M.; Barbieri, S.; Sottotetti, F.; La Rovere, M.T.; Gabanelli, P. Mental health and risk perception among Italian healthcare workers during the second month of the COVID-19 pandemic. Arch. Psychiatr. Nurs. 2020, 34, 537–544. [Google Scholar] [CrossRef]
- Vanni, G.; Pellicciaro, M.; Materazzo, M.; Palombi, L.; Buonomo, O.C. Breast Cancer Diagnosis in Coronavirus-Era: Alert from Italy. Front. Oncol. 2020, 22, 938. [Google Scholar] [CrossRef]
- Casella, D.; Fusario, D.; Cassetti, D.; Miccoli, S.; Pesce, A.L.; Bernini, A.; Neri, A. The patient’s pathway for breast cancer in the COVID-19 era: An Italian single-center experience. Breast J. 2020, 26, 1589–1592. [Google Scholar] [CrossRef]
- Veronesi, P.; Corso, G. Impact of COVID-19 pandemic on clinical and surgical breast cancer management. eClinicalMedicine 2020, 26, 100523. [Google Scholar] [CrossRef]
- Toss, A.; Isca, C.; Venturelli, M.; Nasso, C.; Ficarra, G.; Bellelli, V.; Armocida, C.; Barbieri, E.; Cortesi, L.; Moscetti, L. Two-month stop in mammographic screening significantly impacts on breast cancer stage at diagnosis and upfront treatment in the COVID era. ESMO Open 2021, 6, 100055. [Google Scholar] [CrossRef]
- Spadea, T.; Di Girolamo, C.; Landriscina, T.; Leoni, O.; Forni, S.; Colais, P.; Gnavi, R. Mimico-19 working group. Indirect impact of COVID-19 on hospital care pathways in Italy. Sci. Rep. 2021, 11, 21526. [Google Scholar] [CrossRef]
- Fortunato, L.; d’Amati, G.; Taffurelli, M.; Tinterri, C.; Marotti, L.; Cataliotti, L. Severe Impact of COVID-19 Pandemic on Breast Cancer Care in Italy: A Senonetwork National Survey. Clin. Breast Cancer 2021, 21, e165–e167. [Google Scholar] [CrossRef]
- Richards, M.; Anderson, M.; Carter, P.; Ebert, L.B.; Mossialos, E. The impact of the COVID-19 pandemic on cancer care. Nat. Cancer 2020, 1, 565–567. [Google Scholar] [CrossRef] [PubMed]
- Rusch, V.W.; Wexner, S.D.; Atala, A.; Atkinson, J.L.; Denneny, J.C., III; Eberlein, T.J.; Wood, D.E. The American College of Surgeons Responds to COVID-19. J. Am. Coll. Surg. 2020, 231, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Thornton, J. Clinical trials suspended in UK to prioritise COVID-19 studies and free up staff. BMJ 2020, 368, m1172. [Google Scholar] [CrossRef]
- Ndumele, A.; Park, K.U. The Impact of COVID-19 on National Clinical Trials Network Breast Cancer Trials. Curr. Breast Cancer Rep. 2021, 13, 103–109. [Google Scholar] [CrossRef]
- Rocco, N.; Montagna, G.; Di Micco, R.; Benson, J.; Criscitiello, C.; Chen, L.; Di Pace, B.; Esgueva Colmenarejo, A.J.; Harder, Y.; Karakatsanis, A.; et al. The Impact of the COVID-19 Pandemic on Surgical Management of Breast Cancer: Global Trends and Future Perspectives. Oncologist 2021, 26, e66–e77. [Google Scholar] [CrossRef]
- Magno, S.; Linardos, M.; Carnevale, S.; Dilucca, M.; Di Leone, A.; Terribile, D.A.; Franceschini, G.; Masetti, R. The impact of the COVID-19 pandemic on breast cancer patients awaiting surgery: Observational survey in an Italian University hospital. Breast J. 2020, 26, 1597–1602. [Google Scholar] [CrossRef] [PubMed]
- Vanni, G.; Pellicciaro, M.; Combi, F.; Papi, S.; Materazzo, M.; Segattini, S.; Rizza, S.; Chiocchi, M.; Perretta, T.; Meucci, R.; et al. Impact of COVID-19 Pandemic on Surgical Breast Cancer Patients Undergoing Neoadjuvant Therapy: A Multicentric Study. Anticancer Res. 2021, 41, 4535–4542. [Google Scholar] [CrossRef] [PubMed]
- Vissio, E.; Falco, E.C.; Collemi, G.; Borella, F.; Papotti, M.; Scarmozzino, A.; Cassoni, P.; Bertero, L. Impact of COVID-19 lockdown measures on oncological surgical activity: Analysis of the surgical pathology caseload of a tertiary referral hospital in Northwestern Italy. J. Surg. Oncol. 2021, 123, 24–31. [Google Scholar] [CrossRef]
- WHO; Classification of Tumours Editorial Board. Breast Tumours, 5th ed.; WHO Classification of Tumours Series; International Agency for Research on Cancer: Lyon, France, 2019; Volume 2.
- Duffy, S.W.; Tabar, L.; Vitak, B.; Warwick, J. Tumor size and breast cancer detection: What might be the effect of a less sensitive screening tool than mammography? Breast J. 2006, 12, S91–S95. [Google Scholar] [CrossRef]
- Castellano, I.; Chiusa, L.; Vandone, A.M.; Beatrice, S.; Goia, M.; Donadio, M.; Arisio, R.; Muscara, F.; Durando, A.; Viale, G.; et al. A simple and reproducible prognostic index in luminal ER-positive breast cancers. Ann. Oncol. 2013, 24, 2292–2297. [Google Scholar] [CrossRef]
- Vissio, E.; Metovic, J.; Osella-Abate, S.; Bertero, L.; Migliaretti, G.; Borella, F.; Benedetto, C.; Sapino, A.; Cassoni, P.; Castellano, I. Integration of Ki-67 index into AJCC 2018 staging provides additional prognostic information in breast tumours candidate for genomic profiling. Br. J. Cancer 2020, 122, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Goldhirsch, A.; Winer, E.P.; Coates, A.S.; Gelber, R.D.; Piccart-Gebhart, M.; Thürlimann, B.; Senn, H.-J. Personalizing the treatment of women with early breast cancer: Highlights of the st gallen international expert consensus on the primary therapy of early breast Cancer 2013. Ann. Oncol. 2013, 24, 2206–2223. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.H.; Hicks, D.G.; Dowsett, M.; McShane, L.M.; Allison, K.H.; Allred, D.C.; Bartlett, J.M.S.; Bilous, M.; Fitzgibbons, P.; et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Update. J. Clin. Oncol. 2013, 31, 3997–4013. [Google Scholar] [CrossRef] [PubMed]
- Goldhirsch, A.; Wood, W.C.; Coates, A.S.; Gelber, R.D.; Thürlimann, B.; Senn, H.-J.; Panel members. Strategies for subtypes-dealing with the diversity of breast cancer: Highlights of the St Gallen international expert consensus on the primary therapy of early breast cancer 2011. Ann. Oncol. 2011, 22, 1736–1747. [Google Scholar] [CrossRef] [PubMed]
- Bustreo, S.; Osella-Abate, S.; Cassoni, P.; Donadio, M.; Airoldi, M.; Pedani, F.; Papotti, M.; Sapino, A.; Castellano, I. Optimal Ki67 cut-off for luminal breast cancer prognostic evaluation: A large case series study with a long-term follow-up. Breast Cancer Res. Treat. 2016, 157, 363–371. [Google Scholar] [CrossRef]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours, 8th ed.; John Wiley and Sons: Chichester, UK, 2017. [Google Scholar]
- Jazieh, A.R.; Akbulut, H.; Curigliano, G.; Rogado, A.; Alsharm, A.A.; Razis, E.D.; Mula-Hussain, L.; Errihani, H.; Khattak, A.; De Guzman, R.B.; et al. International Research Network on COVID-19 Impact on Cancer Care. Impact of the COVID-19 Pandemic on Cancer Care: A Global Collaborative Study. JCO Glob. Oncol. 2020, 6, 1428–1438. [Google Scholar] [CrossRef]
- Kaufman, H.W.; Chen, Z.; Niles, J.; Fesko, Y. Changes in the number of US patients with newly identified cancer before and during the Coronavirus disease 2019 (COVID-19) pandemic. JAMA Netw. Open 2020, 3, e2017267. [Google Scholar] [CrossRef]
- Vanni, G.; Pellicciaro, M.; Materazzo, M.; Bruno, V.; Oldani, C.; Pistolese, C.A.; Buonomo, C.; Caspi, J.; Gualtieri, P.; Chiaravalloti, A.; et al. Lockdown of Breast Cancer Screening for COVID-19: Possible Scenario. In Vivo 2020, 34, 3047–3053. [Google Scholar] [CrossRef]
- Vanni, G.; Tazzioli, G.; Pellicciaro, M.; Materazzo, M.; Paolo, O.; Cattadori, F.; Combi, F.; Papi, S.; Pistolese, C.A.; Cotesta, M.; et al. Delay in Breast Cancer Treatments During the First COVID-19 Lockdown. A Multicentric Analysis of 432 Patients. Anticancer Res. 2020, 40, 7119–7125. [Google Scholar] [CrossRef] [PubMed]
- Mentrasti, G.; Cantini, L.; Vici, P.; D’Ostilio, N.; La Verde, N.; Chiari, R.; Paolucci, V.; Crocetti, S.; De Filippis, C.; Pecci, F.; et al. Rising incidence of late stage breast cancer after COVID-19 outbreak. Real-world data from the Italian COVID-DELAY study. Breast 2022, 65, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Bleicher, R.J. Timing and Delays in Breast Cancer Evaluation and Treatment. Ann. Surg. Oncol. 2018, 25, 2829–2838. [Google Scholar] [CrossRef]
- Xu, J.; Bromley, L.; Chew, G.; Yeo, B. “First Do No Harm”: Significance of Delays from Diagnosis to Surgery in Patients with Non-metastatic Breast Cancer. World J. Surg. 2020, 44, 3812–3820. [Google Scholar] [CrossRef] [PubMed]
- Losurdo, A.; Lisa, A.V.E.; Tomatis, M.; Ponti, A.; Montemezzi, S.; Bonzano, E.; Fortunato, L. Senonetwork Working Group. Highly specialized Breast Centers did not experience delay of care during COVID-19 pandemic in Italy: The Senonetwork experience. Breast Cancer Res Treat. 2022, 196, 87–95. [Google Scholar] [CrossRef]
- Giuliano, A.E.; Ballman, K.V.; McCall, L.; Beitsch, P.D.; Brennan, M.B.; Kelemen, P.R.; Ollila, D.W.; Hansen, N.M.; Whitworth, P.W.; Blumencranz, P.W.; et al. Effect of axillary dissection vs no axillary dissection on 10-Year overall survival among women with invasive breast cancer and sentinel node metastasis: The ACOSOG Z0011 (Alliance) randomized clinical trial. JAMA 2017, 318, 918. [Google Scholar] [CrossRef]
- Magnoni, F.; Galimberti, V.; Corso, G.; Intra, M.; Sacchini, V.; Veronesi, P. Axillary surgery in breast cancer: An updated historical perspective. Semin. Oncol. 2020, 47, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.Y.; Santa-Maria, C.A.; Mangini, N.; Norman, H.; Couzi, R.; Nunes, R.; Wilkinson, M.; Visvanathan, K.; Connolly, R.M.; Torres, E.T.R.; et al. Management of Breast Cancer During the COVID-19 Pandemic: A Stage- and Subtype-Specific Approach. JCO Oncol. Pract. 2020, 16, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Korde, L.A.; Somerfield, M.R.; Carey, L.A.; Crews, J.R.; Denduluri, N.; Hwang, E.S.; Khan, S.A.; Loibl, S.; Morris, E.A.; Perez, A.; et al. Neoadjuvant Chemotherapy, Endocrine Therapy, and Targeted Therapy for Breast Cancer: ASCO Guideline. J. Clin. Oncol. 2021, 39, 1485–1505. [Google Scholar] [CrossRef]
- Dietz, J.R.; Moran, M.S.; Isakoff, S.J.; Kurtzman, S.H.; Willey, S.C.; Burstein, H.J.; Bleicher, R.J.; Lyons, J.A.; Sarantou, T.; Baron, P.L.; et al. Recommendations for prioritization, treatment, and triage of breast cancer patients during the COVID-19 pandemic. the COVID-19 pandemic breast cancer consortium. Breast Cancer Res. Treat. 2020, 181, 487–497. [Google Scholar] [CrossRef]
- Dowsett, M.; Ellis, M.J.; Dixon, J.M.; Gluz, O.; Robertson, J.; Kates, R.; Suman, V.J.; Turnbull, A.K.; Nitz, U.; Christgen, M.; et al. Evidence-based guidelines for managing patients with primary ER+ HER2− breast cancer deferred from surgery due to the COVID-19 pandemic. NPJ Breast Cancer 2020, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.K.; Lee, M.K.; Baker, J.L.; Attai, D.J.; DiNome, M.L. Taking a Second Look at Neoadjuvant Endocrine Therapy for the Treatment of Early Stage Estrogen Receptor Positive Breast Cancer During the COVID-19 Outbreak. Ann. Surg. 2020, 272, e96–e97. [Google Scholar] [CrossRef] [PubMed]
- Park, K.U.; Gregory, M.; Bazan, J.; Lustberg, M.; Rosenberg, S.; Blinder, V.; Sharma, P.; Pusztai, L.; Shen, C.; Partridge, A.; et al. Neoadjuvant endocrine therapy use in early stage breast cancer during the COVID-19 pandemic. Breast Cancer Res. Treat. 2021, 188, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Martí, C.; Sánchez-Méndez, J.I. Neoadjuvant endocrine therapy for luminal breast cancer treatment: A first-choice alternative in times of crisis such as the COVID-19 pandemic. Ecancermedicalscience 2020, 14, 1027. [Google Scholar] [CrossRef] [PubMed]
- Gross, M.W.; Läubli, H.; Cordier, D. Multidisciplinary tumor boards as videoconferences—A new challenge in the COVID-19 era. Ann. Oncol. 2021, 32, 572–573. [Google Scholar] [CrossRef]
- Highton, L.R.; Dave, R.V.; Barnes, N.L.P. Breast cancer surgery during the COVID-19 pandemic. Br. J. Surg. 2020, 107, e380. [Google Scholar] [CrossRef]
- Kara, H.; Arikan, A.E.; Dulgeroglu, O.; Tutar, B.; Tokat, F.; Uras, C. Has the COVID-19 Pandemic Affected Breast Cancer Stage and Surgical Volume? Front. Surg. 2022, 9, 811108. [Google Scholar] [CrossRef] [PubMed]
| Variable | All Patients (N = 1331) |
|---|---|
| Median age (years) SD (range) | 62 ± 13 (25–91) |
| Screening | |
| Yes | 237 (18%) |
| No | 1094 (82%) |
| Median time (days) between radiological diagnosis and surgery (in situ tumors) SD (range) | 69 ± 25 (11–133) |
| Median time (days) between radiological diagnosis and surgery * (excluding NACHT) SD (range) | 67 ± 26 (11–155) |
| Clinical stage at diagnosis | |
| Stage 0–I–II | 1266 (95%) |
| Stage III | 65 (5%) |
| Breast surgery | |
| Conservative | 895 (67%) |
| Mastectomy | 436 (35%) |
| Axillary dissection * | |
| No | 970 (84%) |
| Yes | 178 (16%) |
| Invasiveness | |
| In situ | 183 (14%) |
| Invasive | 1148 (86%) |
| Diameter (mm) * | |
| <15 | 556 (48%) |
| ≥15 | 592 (52%) |
| Grade * | |
| 1 | 270 (27%) |
| 2–3 | 878 (73%) |
| Histotype * | |
| NST | 779 (66%) |
| Lobular | 175 (16%) |
| Others | 194 (18%) |
| Molecular profile * | |
| Luminal A | 635 (54%) |
| Luminal B | 305 (30%) |
| HER2+ HR+ | 69 (5%) |
| HER2+ HR− | 28 (2%) |
| Triple-negative | 111 (9%) |
| NACHT * | |
| No | 1026 (77%) |
| Yes | 122 (33%) |
| Pathological T stage (excluding NACHT) * | |
| 1 | 750 (73%) |
| 2 | 229 (22%) |
| 3–4 | 47 (5%) |
| Pathological N stage (excluding NACHT) * | |
| 0 | 408 (74%) |
| 1 | 107 (19%) |
| 2–3 | 40 (7%) |
| Pathological T stage (considering only NACHT) * | |
| 0 | 35 (29%) |
| 1 | 55 (45%) |
| 2 | 26 (21%) |
| 3–4 | 6 (5%) |
| Pathological N stage (considering only NACHT) * | |
| 0 | 71 (58%) |
| 1 | 31 (26%) |
| 2 | 12 (10%) |
| 3 | 8 (6%) |
| Variable | Years 2018–2019 (N = 726) | Years 2020–2021 (N = 605) | p-Value |
|---|---|---|---|
| Median age (years) SD (range) | 61 ± 13 (26–89) | 64 ± 14 (25–91) | 0.002 |
| Screening | |||
| Yes | 133 (18%) | 104 (17%) | 0.529 |
| No | 593 (82%) | 501 (83%) | |
| Median time (days) between radiological diagnosis and surgery (in situ tumors) SD (range) | 67 ± 25 (17–133) | 72 ± 25 (11–136) | 0.238 |
| Median time (days) between radiological diagnosis and surgery * (excluding NACHT) SD (range) | 67 ± 27 (12–154) | 66 (11–155) | 0.66 |
| Clinical stage at diagnosis | |||
| Stage 0–I–II | 688 (95%) | 578 (96%) | 0.56 |
| Stage III | 38 (5%) | 27 (4%) | |
| Breast surgery | |||
| Conservative | 490 (67%) | 405 (67%) | 0.831 |
| Mastectomy | 236 (33%) | 200 (33%) | |
| Axillary dissection | |||
| No | 488 (79%) | 482 (92%) | <0.001 |
| Yes | 136 (21%) | 42 (8%) | |
| Invasiveness | |||
| In situ | 102 (14%) | 81 (13%) | 0.727 |
| Invasive | 624 (86%) | 524 (87%) | |
| Diameter (mm) * | |||
| <15 | 299 (48%) | 257 (49%) | 0.703 |
| ≥15 | 325 (52%) | 267 (51%) | |
| Grade * | |||
| 1 | 166 (27%) | 104 (20%) | 0.007 |
| 2–3 | 458 (73%) | 420 (80%) | |
| Histotype * | |||
| NST | 412 (66%) | 367 (70%) | 0.266 |
| Lobular | 97 (16%) | 78 (15%) | |
| Others | 115 (18%) | 79 (15%) | |
| Molecular profile * | |||
| Luminal A | 337 (54%) a | 298 (57%) a | 0.015 |
| Luminal B | 188 (30%) a | 117 (22%) b | |
| HER2+ HR+ | 31 (5%) a | 38 (7%) a | |
| HER2+ HR− | 11 (2%) a | 17 (3%) a | |
| Triple-negative | 57 (9%) a | 54 (10%) a | |
| NACHT * | |||
| No | 555 (89%) | 471 (90%) | 0.606 |
| Yes | 69 (11%) | 53 (10%) | |
| Pathological T stage (excluding NACHT) * | |||
| 1 | 407 (73%) a | 343 (73%) a | 0.03 |
| 2 | 131 (24%) a | 98 (21%) a | |
| 3–4 | 17 (3%) a | 30 (6%) b | |
| Pathological N stage (excluding NACHT) * | |||
| 0 | 408 (74%) | 363 (77%) | 0.411 |
| 1 | 107 (19%) | 80 (17%) | |
| 2–3 | 40 (7%) | 28 (6%) | |
| Pathological T stage (considering only NACHT) * | |||
| 0 | 16 (23%) | 19 (36%) | 0.422 |
| 1 | 32 (46%) | 23 (43%) | |
| 2 | 17 (25%) | 9 (17%) | |
| 3–4 | 4 (6%) | 2 (4%) | |
| Pathological N stage (considering only NACHT) * | |||
| 0 | 36 (52%) | 35 (66%) | 0.410 |
| 1 | 19 (27%) | 12 (23%) | |
| 2 | 8 (12%) | 4 (7%) | |
| 3 | 6 (9%) | 2 (4%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borella, F.; Bertero, L.; Di Giovanni, F.; Witel, G.; Orlando, G.; Ricci, A.A.; Pittaro, A.; Castellano, I.; Cassoni, P. COVID-19 and Breast Cancer: Analysis of Surgical Management of a Large Referral Center during the 2020–2021 Pandemic Period. Curr. Oncol. 2023, 30, 4767-4778. https://doi.org/10.3390/curroncol30050359
Borella F, Bertero L, Di Giovanni F, Witel G, Orlando G, Ricci AA, Pittaro A, Castellano I, Cassoni P. COVID-19 and Breast Cancer: Analysis of Surgical Management of a Large Referral Center during the 2020–2021 Pandemic Period. Current Oncology. 2023; 30(5):4767-4778. https://doi.org/10.3390/curroncol30050359
Chicago/Turabian StyleBorella, Fulvio, Luca Bertero, Fabrizia Di Giovanni, Gianluca Witel, Giulia Orlando, Alessia Andrea Ricci, Alessandra Pittaro, Isabella Castellano, and Paola Cassoni. 2023. "COVID-19 and Breast Cancer: Analysis of Surgical Management of a Large Referral Center during the 2020–2021 Pandemic Period" Current Oncology 30, no. 5: 4767-4778. https://doi.org/10.3390/curroncol30050359