Efficacy and Safety of Monacolin K Combined with Coenzyme Q10, Grape Seed, and Olive Leaf Extracts in Improving Lipid Profile of Patients with Mild-to-Moderate Hypercholesterolemia: A Self-Control Study
Abstract
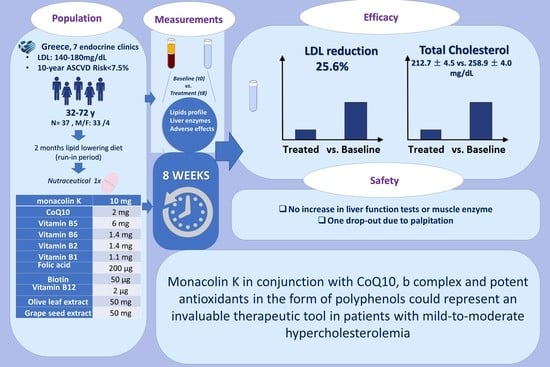
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Measurements
2.3. Statistical Analysis
3. Results
4. Discussion
4.1. RYR-Based Supplements Efficacy
4.2. Safety of RYR-Based Supplements
4.3. Efficacy of GSE, Polyphenols, and Vitamin B Supplements
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALT | Alanine aminotransferase |
| ANSES | French Agency for Food, Environmental and Occupational Health & Safety |
| AST | aspartate aminotransferase |
| ASCVD | Atherosclerotic cardiovascular disease |
| BMI | Body mass index |
| BP | Blood pressure |
| Co Q10 | Coenzyme Q10 |
| CHD | Coronary heart disease |
| CK | creatine kinase |
| CYP3A4 | Cytochrome P450 3A4 |
| γ-gt | gamma-glutamyl transferase FDA— Food and Drug Administration |
| GSE | grape seed extract |
| HDL-C | High-density lipoprotein cholesterol |
| HgbA1c | Hemoglobin A1c |
| HMG-CoA | hydroxymethylglutaryl-coenzyme A |
| LDL-C | Low-density lipoprotein cholesterol |
| MDRD GFR | Modification of Diet in Renal Disease calculated glomerular filtration rate |
| OLE | Olive leaf extract |
| RYR | Red yeast rice extract |
| SAMS | Statin-associated muscle symptoms |
| TC | Total cholesterol |
| TG | Triglycerides |
| WHO | World Health Organization |
References
- Endo, A. A gift from nature: The birth of the statins. Nat. Med. 2008, 14, 1050–1052. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.W.; Mousa, S.A. The effect of red yeast rice (Monascus purpureus) in dyslipidemia and other disorders. Complement. Ther. Med. 2012, 20, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.; Derosa, G.; Parini, A.; Maffioli, P.; D’Addato, S.; Reggi, A.; Giovannini, M.; Borghi, C. Red yeast rice improves lipid pattern, high-sensitivity C-reactive protein, and vascular remodeling parameters in moderately hypercholesterolemic Italian subjects. Nutr. Res. 2013, 33, 622–628. [Google Scholar] [CrossRef]
- Solà, R.; Valls, R.M.; Puzo, J.; Calabuig, J.-R.; Brea, A.; Pedret, A.; Moriña, D.; Villar, J.; Millán, J.; Anguera, A. Effects of Poly-Bioactive Compounds on Lipid Profile and Body Weight in a Moderately Hypercholesterolemic Population with Low Cardiovascular Disease Risk: A Multicenter Randomized Trial. PLoS ONE 2014, 9, e101978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cicero, A.F.; Colletti, A. Combinations of phytomedicines with different lipid lowering activity for dyslipidemia management: The available clinical data. Phytomedicine 2016, 23, 1113–1118. [Google Scholar] [CrossRef]
- Kumar, A.; Kaur, H.; Devi, P.; Mohan, V. Role of coenzyme Q10 (CoQ10) in cardiac disease, hypertension and Meniere-like syndrome. Pharmacol. Ther. 2009, 124, 259–268. [Google Scholar] [CrossRef] [PubMed]
- El, S.N.; Karakaya, S. Olive tree (Olea europaea) leaves: Potential beneficial effects on human health. Nutr. Rev. 2009, 67, 632–638. [Google Scholar] [CrossRef]
- Silva, S.; Gomes, L.; Leitão, F.; Coelho, A.V.; Boas, L.V. Phenolic Compounds and Antioxidant Activity of Olea europaea L. Fruits and Leaves. Food Sci. Technol. Int. 2006, 12, 385–395. [Google Scholar] [CrossRef]
- Gariboldi, P.; Jommi, G.; Verotta, L. Secoiridoids from Olea europaea. Phytochemistry 1986, 25, 865–869. [Google Scholar] [CrossRef]
- Hadrich, F.; Mahmoudi, A.; Bouallagui, Z.; Feki, I.; Isoda, H.; Feve, B.; Sayadi, S. Evaluation of hypocholesterolemic effect of oleuropein in cholesterol-fed rats. Chem. Interact. 2016, 252, 54–60. [Google Scholar] [CrossRef]
- Priore, P.; Siculella, L.; Gnoni, G.V. Extra virgin olive oil phenols down-regulate lipid synthesis in primary-cultured rat-hepatocytes. J. Nutr. Biochem. 2014, 25, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Dohadwala, M.M.; Vita, J.A. Grapes and Cardiovascular Disease. J. Nutr. 2009, 139, 1788S–1793S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shinagawa, F.B.; De Santana, F.C.; Torres, L.R.O.; Mancini-Filho, J. Grape seed oil: A potential functional food? Food Sci. Technol. 2015, 35, 399–406. [Google Scholar] [CrossRef] [Green Version]
- Ueland, P.M.; Loscalzo, J. Homocysteine and Cardiovascular Risk: The Perils of Reductionism in a Complex System. Clin. Chem. 2012, 58, 1623–1625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rimm, E.B.; Willett, W.C.; Hu, F.B.; Sampson, L.; Colditz, G.A.; Manson, J.E.; Hennekens, C.; Stampfer, M.J. Folate and Vitamin B6 From Diet and Supplements in Relation to Risk of Coronary Heart Disease Among Women. JAMA 1998, 279, 359–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voutilainen, S.; Rissanen, T.H.; Virtanen, J.; Lakka, T.; Salonen, J.T. Low Dietary Folate Intake Is Associated With an Excess Incidence of Acute Coronary Events. Circulation 2001, 103, 2674–2680. [Google Scholar] [CrossRef] [Green Version]
- Bønaa, K.H.; Njølstad, I.; Ueland, P.M.; Schirmer, H.; Tverdal, A.; Steigen, T.; Wang, H.; Nordrehaug, J.E.; Arnesen, E.; Rasmussen, K. Homocysteine Lowering and Cardiovascular Events after Acute Myocardial Infarction. N. Engl. J. Med. 2006, 354, 1578–1588. [Google Scholar] [CrossRef] [Green Version]
- Lonn, E.; Yusuf, S.; Arnold, M.J.; Sheridan, P.; Pogue, J.; Micks, M.; McQueen, M.J.; Probstfield, J.; Fodor, G.; Held, C.; et al. Homocysteine Lowering with Folic Acid and B Vitamins in Vascular Disease. N. Engl. J. Med. 2006, 354, 1567–1577. [Google Scholar] [CrossRef] [Green Version]
- Hankey, G.J. B vitamins in patients with recent transient ischaemic attack or stroke in the VITAmins TO Prevent Stroke (VITATOPS) trial: A randomised, double-blind, parallel, placebo-controlled trial. Lancet Neurol. 2010, 9, 855–865. [Google Scholar] [CrossRef] [Green Version]
- Cicero, A.F.G.; Colletti, A.; Bajraktari, G.; Descamps, O.; Djuric, D.M.; Ezhov, M.; Fras, Z.; Katsiki, N.; Langlois, M.; Latkovskis, G.; et al. Lipid-lowering nutraceuticals in clinical practice: Position paper from an International Lipid Expert Panel. Nutr. Rev. 2017, 75, 731–767. [Google Scholar] [CrossRef]
- Gerards, M.C.; Terlou, R.J.; Yu, H.; Koks, C.; Gerdes, V. Traditional Chinese lipid-lowering agent red yeast rice results in significant LDL reduction but safety is uncertain—A systematic review and meta-analysis. Atherosclerosis 2015, 240, 415–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cicero, A.F.; Parini, A.; Rosticci, M. Nutraceuticals and cholesterol-lowering action. IJC Metab. Endocr. 2015, 6, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Banach, M.; Patti, A.M.; Giglio, R.V.; Cicero, A.F.; Atanasov, A.G.; Bajraktari, G.; Bruckert, E.; Descamps, O.; Djuric, D.M.; Ezhov, M.; et al. The Role of Nutraceuticals in Statin Intolerant Patients. J. Am. Coll. Cardiol. 2018, 72, 96–118. [Google Scholar] [CrossRef] [PubMed]
- Dujovne, C.A.; Chremos, A.; Pool, J.L.; Schnaper, H.; Bradford, R.H.; Shear, C.L.; Higgins, J.; Downton, M.; Franklin, F.A.; Nash, D.T.; et al. Expanded clinical evaluation of lovastatin (EXCEL) study results: IV. Additional perspectives on the tolerability of lovastatin. Am. J. Med. 1991, 91, S25–S30. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, Y.; Chiriboga, D.E.; Olendzki, B.C.; Hebert, J.R.; Li, W.; Leung, K.; Hafner, A.R.; Ockene, I.S. Association between Carbohydrate Intake and Serum Lipids. J. Am. Coll. Nutr. 2006, 25, 155–163. [Google Scholar] [CrossRef] [Green Version]
- Furberg, C.D.; Adams, H.P.; Applegate, W.B.; Byington, R.P.; Espeland, M.A.; Hartwell, T.; Hunninghake, D.B.; Lefkowitz, D.S.; Probstfield, J.; Riley, W.A. Effect of lovastatin on early carotid atherosclerosis and cardiovascular events. Asymptomatic Carotid Artery Progression Study (ACAPS) Research Group. Circulation 1994, 90, 1679–1687. [Google Scholar] [CrossRef] [Green Version]
- Weintraub, W.S.; Boccuzzi, S.J.; Klein, J.L.; Kosinski, A.S.; King, S.B.; Ivanhoe, R.; Cedarholm, J.C.; Stillabower, M.E.; Talley, J.D.; DeMaio, S.J.; et al. Lack of Effect of Lovastatin on Restenosis after Coronary Angioplasty. N. Engl. J. Med. 1994, 331, 1331–1337. [Google Scholar] [CrossRef]
- Downs, J.R.; Clearfield, M.; Tyroler, H.; Whitney, E.J.; Kruyer, W.; Langendorfer, A.; Zagrebelsky, V.; Weis, S.; Shapiro, D.R.; Beere, P.A.; et al. Air force/texas coronary atherosclerosis prevention study (afcaps/texcaps): Additional perspectives on tolerability of long-term treatment with lovastatin. Am. J. Cardiol. 2001, 87, 1074–1079. [Google Scholar] [CrossRef]
- Mazzanti, G.; Moro, P.A.; Raschi, E.; Da Cas, R.; Menniti-Ippolito, F. Adverse reactions to dietary supplements containing red yeast rice: Assessment of cases from the Italian surveillance system. Br. J. Clin. Pharmacol. 2017, 83, 894–908. [Google Scholar] [CrossRef]
- Grieco, A.; Miele, L.; Pompili, M.; Biolato, M.; Vecchio, F.M.; Grattagliano, I.; Gasbarrini, G. Acute hepatitis caused by a natural lipid-lowering product: When “alternative” medicine is no “alternative” at all. J. Hepatol. 2009, 50, 1273–1277. [Google Scholar] [CrossRef]
- Lapi, F.; Gallo, E.; Bernasconi, S.; Vietri, M.; Menniti-Ippolito, F.; Raschetti, R.; Gori, L.; Firenzuoli, F.; Mugelli, A.; Vannacci, A. Myopathies associated with red yeast rice and liquorice: Spontaneous reports from the Italian Surveillance System of Natural Health Products. Br. J. Clin. Pharmacol. 2008, 66, 572–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anjom-Shoae, J.; Milajerdi, A.; Larijani, B.; Esmaillzadeh, A. Effects of grape seed extract on dyslipidaemia: A systematic review and dose–response meta-analysis of randomised controlled trials. Br. J. Nutr. 2020, 124, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Mazza, A.; Schiavon, L.; Rigatelli, G.; Torin, G.; Montanaro, F.; Lenti, S. The short-term supplementation of monacolin K improves the lipid and metabolic patterns of hypertensive and hypercholesterolemic subjects at low cardiovascular risk. Food Funct. 2018, 9, 3845–3852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feuerstein, J.S.; Bjerke, W.S. Powdered Red Yeast Rice and Plant Stanols and Sterols to Lower Cholesterol. J. Diet. Suppl. 2012, 9, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.; Fogacci, F.; Rosticci, M.; Parini, A.; Giovannini, M.; Veronesi, M.; D’Addato, S.; Borghi, C. Effect of a short-term dietary supplementation with phytosterols, red yeast rice or both on lipid pattern in moderately hypercholesterolemic subjects: A three-arm, double-blind, randomized clinical trial. Nutr. Metab. 2017, 14, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lockyer, S.; Rowland, I.; Spencer, J.P.E.; Yaqoob, P.; Stonehouse, W. Impact of phenolic-rich olive leaf extract on blood pressure, plasma lipids and inflammatory markers: A randomised controlled trial. Eur. J. Nutr. 2016, 56, 1421–1432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Li, H.; Zhou, Y.; Jin, L.; Liu, J. Low-dose B vitamins supplementation ameliorates cardiovascular risk: A double-blind randomized controlled trial in healthy Chinese elderly. Eur. J. Nutr. 2014, 54, 455–464. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Z.; Liu, S.; Liu, Y.; Ma, Y.; Liu, Y.; Xue, M.; Li, Q.; Zhang, X.; Zhang, S.; et al. Effect of B vitamins supplementation on cardio-metabolic factors in patients with stable coronary artery disease: A randomized double-blind trial. Asia Pac. J. Clin. Nutr. 2020, 29, 245–252. [Google Scholar]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Atherosclerosis 2019, 290, 140–205. [Google Scholar] [CrossRef] [Green Version]
- Raschi, E.; Girardi, A.; Poluzzi, E.; Forcesi, E.; Menniti-Ippolito, F.; Mazzanti, G.; De Ponti, F. Adverse Events to Food Supplements Containing Red Yeast Rice: Comparative Analysis of FAERS and CAERS Reporting Systems. Drug Saf. 2018, 41, 745–752. [Google Scholar] [CrossRef]
- Goff, D.C., Jr.; Lloyd-Jones, D.M.; Bennett, G.; Coady, S.; D’Agostino, R.B.; Gibbons, R.; Greenland, P.; Lackland, D.T.; Levy, D.; O’Donnell, C.J.; et al. 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk. Circulation 2014, 129 (Suppl. S2), S49–S73. [Google Scholar] [CrossRef] [PubMed]
- Sandesara, P.B.; Virani, S.S.; Fazio, S.; Shapiro, M.D. The Forgotten Lipids: Triglycerides, Remnant Cholesterol, and Atherosclerotic Cardiovascular Disease Risk. Endocr. Rev. 2018, 40, 537–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoogeveen, R.C.; Ballantyne, C.M. Residual Cardiovascular Risk at Low LDL: Remnants, Lipoprotein(a), and Inflammation. Clin. Chem. 2020, 67, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. Circulation 2019, 139, e1082–e1143. [Google Scholar] [CrossRef]
- Cicero, A.F.; Fogacci, F.; Zambon, A. Red Yeast Rice for Hypercholesterolemia: JACC Focus Seminar. J. Am. Coll. Cardiol. 2021, 77, 620–628. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Baker, S.K.; Jacobson, T.A.; Kopecky, S.L.; Parker, B.A. An assessment by the Statin Muscle Safety Task Force: 2014 update. J. Clin. Lipidol. 2014, 8, S58–S71. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, C.; Köller, Y.; Surkova, E. Effect of Coenzyme Q10 on statin-associated myalgia and adherence to statin therapy: A systematic review and meta-analysis. Atherosclerosis 2020, 299, 1–8. [Google Scholar] [CrossRef]
- Qu, H.; Guo, M.; Chai, H.; Wang, W.; Gao, Z.; Shi, D. Effects of Coenzyme Q10 on Statin-Induced Myopathy: An Updated Meta-Analysis of Randomized Controlled Trials. J. Am. Hear. Assoc. 2018, 7, e009835. [Google Scholar] [CrossRef] [Green Version]
- Lloyd-Jones, D.; Huffman, M.; Karmali, K.N.; Sanghavi, D.M.; Wright, J.S.; Pelser, C.; Gulati, M.; Masoudi, F.A.; GoffJr, D.C. Estimating Longitudinal Risks and Benefits From Cardiovascular Preventive Therapies Among Medicare Patients: The Million Hearts Longitudinal ASCVD Risk Assessment Tool: A Special Report From the American Heart Association and American College of Cardiology. Circulation 2017, 135, e793–e813. [Google Scholar] [CrossRef] [Green Version]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J.; Himmelfarb, C.D.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2018, 71, e127–e248. [Google Scholar] [CrossRef]
- Correction to: Systematic Review for the 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e1187. [CrossRef]


| RYR monacolin K | 10 mg |
| CoQ10 | 2 mg |
| Vitamin B5 | 6 mg |
| Vitamin B6 | 1.4 mg |
| Vitamin B2 | 1.4 mg |
| Vitamin B1 | 1.1 mg |
| Folic acid | 200 μg |
| Biotin | 50 μg |
| Vitamin B12 | 2 μg |
| OLE | 50 mg |
| GSE | 50 mg |
| Variables | Patients (N = 37) |
|---|---|
| Age (years) | 55.9 ± 1.5 |
| Sex (M/F, %) | 11/89 |
| BMI (Kg/m2) | 25.3 ± 0.54 |
| TC (mg/dL) | 258.9 ± 0.4 |
| LDL-C (mg/dL) | 173.8 ± 3.45 |
| HDL-C (mg/dL) | 63.1 ± 2.6 |
| TG (mg/dL) | 127 ± 12.19 |
| AST (U/L) | 27.43 ± 0.99 |
| ALT (U/L) | 30.43 ± 0.99 |
| γ-gt (U/L) | 29.18 ± 1.09 |
| CPK (IU/L) | 75.89 ± 3.22 |
| Basal | Treated | |
|---|---|---|
| BMI † | 25.3 ± 0.54 | 25.2 ± 0.57 |
| TC † ** | 258.9 ± 4 | 212.7 ± 4.5 |
| HDL-C † | 63.1 ± 2.6 | 63.3 ± 2.5 |
| LDL-C † ** | ||
| Absolute values | 173.8 ± 3.45 | 129 ± 4.53 |
| Difference | −42.35 ± 4.15 | |
| TG ‡ * | 127 ± 12.19 | 117 ± 9.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angelopoulos, N.; Paparodis, R.D.; Androulakis, I.; Boniakos, A.; Anagnostis, P.; Tsimihodimos, V.; Livadas, S. Efficacy and Safety of Monacolin K Combined with Coenzyme Q10, Grape Seed, and Olive Leaf Extracts in Improving Lipid Profile of Patients with Mild-to-Moderate Hypercholesterolemia: A Self-Control Study. Nutraceuticals 2023, 3, 1-12. https://doi.org/10.3390/nutraceuticals3010001
Angelopoulos N, Paparodis RD, Androulakis I, Boniakos A, Anagnostis P, Tsimihodimos V, Livadas S. Efficacy and Safety of Monacolin K Combined with Coenzyme Q10, Grape Seed, and Olive Leaf Extracts in Improving Lipid Profile of Patients with Mild-to-Moderate Hypercholesterolemia: A Self-Control Study. Nutraceuticals. 2023; 3(1):1-12. https://doi.org/10.3390/nutraceuticals3010001
Chicago/Turabian StyleAngelopoulos, Nicholas, Rodis D. Paparodis, Ioannis Androulakis, Anastasios Boniakos, Panagiotis Anagnostis, Vasilis Tsimihodimos, and Sarantis Livadas. 2023. "Efficacy and Safety of Monacolin K Combined with Coenzyme Q10, Grape Seed, and Olive Leaf Extracts in Improving Lipid Profile of Patients with Mild-to-Moderate Hypercholesterolemia: A Self-Control Study" Nutraceuticals 3, no. 1: 1-12. https://doi.org/10.3390/nutraceuticals3010001