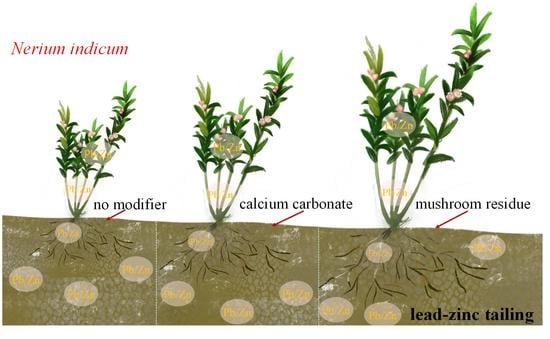
Comparison of Phytoremediation Potential of Nerium indicum with Inorganic Modifier Calcium Carbonate and Organic Modifier Mushroom Residue to Lead–Zinc Tailings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Design
2.3. Determination Method
2.3.1. Physical and Chemical Properties of Tailing
2.3.2. Biomass Increment, Enrichment, and Transport of Heavy Metals of Plants
2.3.3. Data Analysis
3. Results
3.1. Effects of Different Modifiers and Their Gradients on the Basic Characteristics of Tailing
3.1.1. Effects of Different Modifiers and Their Gradients on the pH of Tailing
3.1.2. Effects of Different Modifiers and Their Gradients on the Organic Matter of Tailing
3.1.3. Effects of Different Modifiers and Their Gradients on Soil Enzyme Activity
3.2. Effects of Different Modifiers and Their Gradients on the Morphology of Heavy Metals in Tailings
3.3. Effects of Different Modifiers and Their Gradients on Growth and Tolerance of Nerium indicum
3.3.1. Effects of Different Modifiers and Their Gradients on Nerium indicum Biological Increment
3.3.2. Effects on Heavy Metal Content and Accumulation in Each Part of Nerium indicum
3.3.3. Effects on Bioconcentration Factor, Translocation Factor, and Transfer Factor of Nerium indicum
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Z.; Deblon, J.; Zu, Y.; Colinet, G.; Li, B.; He, Y. Geochemical baseline values determination and evaluation of heavy metal contamination in soils of lanping mining valley (Yunnan Province, China). Int. J. Environ. Res. Public Health 2019, 16, 4686. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ren, Q.; Ge, S.; Jiao, Z.; Zhan, W.; Hou, R.; Ruan, X.; Pan, Y.; Wang, Y. Metal (loid) s spatial distribution, accumulation, and potential health risk assessment in soil-wheat systems near a Pb/Zn smelter in henan province, central china. Int. J. Environ. Res. Public Health 2022, 19, 2527. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Tang, C.; Hao, Y.; Wang, Z.; Yang, G.; Wang, Y.; Mu, Y. Solidification/stabilization of heavy metals and its efficiency in lead-zinc tailings using different chemical agents. Environ. Technol. 2020, 43, 1613–1623. [Google Scholar] [CrossRef] [PubMed]
- Karaca, O.; Cameselle, C.; Reddy, K.R. Mine tailing disposal sites: Contamination problems, remedial options and phytocaps for sustainable remediation. Rev. Environ. Sci. Bio/Technol. 2018, 17, 205–228. [Google Scholar] [CrossRef]
- Wang, P.; Sun, Z.; Hu, Y.; Cheng, H. Leaching of heavy metals from abandoned mine tailings brought by precipitation and the associated environmental impact. Sci. Total Environ. 2019, 695, 133893. [Google Scholar] [CrossRef]
- Yang, L.; Ren, Q.; Zheng, K.; Jiao, Z.; Ruan, X.; Wang, Y. Migration of heavy metals in the soil-grape system and potential health risk assessment. Sci. Total Environ. 2022, 806, 150646. [Google Scholar] [CrossRef]
- Chen, T.; Yan, B.; Lei, C.; Xiao, X. Pollution control and metal resource recovery for acid mine drainage. Hydrometallurgy 2014, 147–148, 112–119. [Google Scholar] [CrossRef]
- Shi, H.B.; Tang, L.; Chen, Y.; Shi, M. Study on the leaching law of lead and zinc in vanadium titanomagnetite tailings. Environ. Sci. Manag. 2020, 45, 68–73. [Google Scholar]
- Shi, Y.N.; Zhan, C.; Zhang, Z.J.; Wu, W.H.; Yang, W. Solidification and stabilization treatment of polluted sludge and planting performance test. Prog. Water Conserv. Hydropower Sci. Technol. 2021, 41, 89–94. [Google Scholar]
- Liu, H.; Zhang, K. Research status and remediation technology prospect of heavy metal pollution in mines. Yunnan Geol. 2018, 37, 117–121. (In Chinese) [Google Scholar]
- Zheng, H.; Ren, Q.; Zheng, K.; Qin, Z.; Wang, Y.; Wang, Y. Spatial distribution and risk assessment of metal(loid)s in marine sediments in the Arctic Ocean and Bering Sea. Mar. Pollut. Bull. 2022, 179, 113729. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.L.; Xiao, H.W.; Cheng, P.G.; Zhao, W.J. Distribution of heavy metal pollution in topsoil and contribution of atmospheric deposition in Beijing. J. Ecol. Environ. 2021, 30, 816–824. [Google Scholar] [CrossRef]
- Han, L.; Chen, Y.; Chen, M.; Wu, Y.; Su, R.; Du, L.; Liu, Z. Mushroom residue modification enhances phytoremediation potential of Paulownia fortunei to lead-zinc slag. Chemosphere 2020, 253, 126774. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Huang, Y.; Wu, W.; Zhong, M.; Xu, F.; Liu, C.; Yu, F. Effect of amendments on fractionation of Pb and Zn in soil. Environ. Chem. 2013, 32, 881–885. [Google Scholar]
- Wang, F.; Zhao, B.; Zhang, F.; Gao, J.; Hao, H.; Zhang, S. A novel heavy metal chelating agent sixthio guanidine acid for in situ remediation of soils contaminated with multielements: Its synthesis, solidification, biodegradability, and leachability. J. Soils Sediments 2016, 16, 371–381. [Google Scholar] [CrossRef]
- Cai, X.; Long, X.; Chong, Y.; Wu, Q. Inorganic-organic amendments for immobilization of metal contaminants in an acidic soil. Acta Sci. Circumstantiae 2015, 35, 3991–4002. [Google Scholar]
- Deng, H.; Li, Z.; Yang, Y.; Xu, Y.; Yang, Y. Leaching of copper and lead in contaminatedlou soil with combined Saponin and EDTA. J. Agro-Environ. Sci. 2015, 34, 461–470. (In Chinese) [Google Scholar]
- Madrid, F.; Florido, M.C. Effects of the presence of a composted biosolid on the metal immobilizing action of an urban soil. J. Hazard. Mater. 2010, 176, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, F.; Wu, X.; Liang, X.; Yuan, S. Effects of different modifier concentrations on lead-zinc tolerance, subcellular distribution and chemical forms for four kinds of woody plants. Environ. Sci. 2015, 36, 3852. [Google Scholar]
- Maier, C.A.; Palmroth, S.; Ward, E. Short-term effects of fertilization on photosynthesis and leaf morphology of field-grown loblolly pine following long-term exposure to elevated CO2 concentration. Tree Physiol. 2008, 28, 597–606. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, S.; Duan, C.; Liu, F.; Li, F. Amelioration of lead-zinc tailings by spent mushroom compost:Effects on growth of lolium perenne L. and physico-chemical properties of tailings. J. Agro-Environ. Sci. 2014, 33, 526–531. [Google Scholar]
- Zhou, H.; Zhou, X.; Zeng, M.; Liao, B.H.; Liu, L.; Yang, W.T.; Wu, Y.M.; Qiu, Q.Y.; Wang, Y.J. Effects of combined amendments on heavy metal accumulation in rice (Oryza sativa L.) planted on contaminated paddy soil. Ecotoxicol. Environ. Saf. 2014, 101, 226–232. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Yang, X.; Stoffella, P. Trace elements in agroecosystems and impacts on the environment. J. Trace Elem. Med. Biol. 2005, 19, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Gao, X.; Zhao, J.; Zhang, J.; Chen, S.; Lu, L. Plant cadmium resistance 2 (SaPCR2) facilitates cadmium efflux in the roots of hyperaccumulator sedum alfredii hance. Front. Plant Sci. 2020, 11, 568887. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Chen, Y.; Wu, T.; Pan, H. Plant growth and metal uptake by Seven Salix clones on Cu/Zn contaminated environment. China Environ. Sci. 2010, 30, 1683–1689. [Google Scholar]
- Yang, L.; Zhu, J.; Wang, P.; Zeng, J.; Tan, R.; Yang, Y.; Liu, Z. Effect of Cd on growth, physiological response, Cd subcellular distribution and chemical forms of Koelreuteria paniculata. Ecotoxicol. Environ. Saf. 2018, 160, 10–18. [Google Scholar] [CrossRef]
- Hu, G.; Yang, X.; Chen, X.; Lu, K.; He, L.; Ye, Z.; Wu, X.; Wang, H. Physiological responses of bamboo-willow plants to heavy metal stress. Acta Sci. Circumstantiae 2016, 36, 3870–3875. [Google Scholar]
- Shang, K.; Zhang, G.; Jiang, Y. The phytoextraction ability of 54 woody species on Cu, Pb, Zn in soil. Chin. J. Ecol. 2019, 38, 3723–3730. [Google Scholar]
- Shi, X.; Chen, Y.; Wang, S.; Li, J. Pb, Zn accumulation and nutrient uptake of 15 plant species grown in abandoned mine tailings. Environ. Sci. 2012, 33, 2021–2027. [Google Scholar]
- Zhao, Z.; Chen, L. Analysis on the application of garden poisonous plants—Taking oleander as an example. China South. Agric. Mach. 2020, 51, 224. (In Chinese) [Google Scholar]
- Zou, T.; Li, T.; Zhang, X.; Yu, H.; Huang, H. Lead accumulation and phytostabilization potential of dominant plant species growing in a lead–zinc mine tailing. Environ. Earth Sci. 2012, 65, 621–630. [Google Scholar] [CrossRef]
- Qian, B.; Liu, L.; Xiao, X. Comparative tests on different methods for content of soil organic matter. J. Hohai Univ. (Nat. Sci.) 2011, 39, 34–38. (In Chinese) [Google Scholar]
- Nemati, K.; Abu Bakar, N.K.; Abas, M.R.; Sobhanzadeh, E. Speciation of heavy metals by modified BCR sequential extraction procedure in different depths of sediments from Sungai Buloh, Selangor, Malaysia. J. Hazard. Mater. 2011, 192, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xu, J.; Shu, R.; Wei, H. Impacts of different intercropping times of garlic on soil enzyme activities in rhizosphere of tomato. Jiangsu Agric. Sci. 2018, 46, 104–107. (In Chinese) [Google Scholar]
- Zhang, S.; Lin, H.; Deng, L.; Gong, G.; Jia, Y.; Xu, X.; Li, T.; Li, Y.; Chen, H. Cadmium tolerance and accumulation characteristics of Siegesbeckia orientalis L. Ecol. Eng. 2013, 51, 133–139. [Google Scholar] [CrossRef]
- He, L.; Su, R.; Chen, Y.; Zeng, P.; Du, L.; Cai, B.; Zhang, A.; Zhu, H. Integration of manganese accumulation, subcellular distribution, chemical forms, and physiological responses to understand manganese tolerance in Macleaya cordata. Environ. Sci. Pollut. Res. 2022, 29, 39017–39026. [Google Scholar] [CrossRef]
- Li, H.; Jia, Y.; Wang, G. Accumulative and transmitting features of the soil-laden lead in tobaccos and its safety threshold for the tobacco-growing soils. J. Saf. Environ. 2014, 14, 305–309. [Google Scholar]
- Jianbin, L.I.; Chen, Y.; Tang, C.; Gong, Z.; Wei, H.E. Plants remediation effects of peat soil on lead-zinc tailings. J. Cent. South Univ. For. Technol. 2019, 39, 93–100. (In Chinese) [Google Scholar]
- Guo, X.; Wei, Z.; Zhou, J.; Chen, H.; Wu, Q. Effect of waste-CaCO3 on heavy metals uptake of low-accumulating maize: Field study. Acta Pedol. Sin. 2010, 47, 888–895. [Google Scholar]
- Chien, S.-W.C.; Wang, H.-H.; Chen, Y.-M.; Wang, M.-K.; Liu, C.-C. Removal of heavy metals from contaminated paddy soils using chemical reductants coupled with dissolved organic carbon solutions. J. Hazard. Mater. 2021, 403, 123549. [Google Scholar] [CrossRef]
- Moghal, A.; Lateef, M.; Mohammed, S.; Lemboye, K.; Chittoori, B.; Almajed, A. Efficacy of enzymatically induced calcium carbonate precipitation in the retention of heavy metal ions. Sustainability 2020, 12, 7019. [Google Scholar] [CrossRef]
- Zeng, C.C.; Hu, H.M.; Feng, X.H.; Wang, K.; Zhang, Q.W. Activating CaCO3 to enhance lead removal from lead-zinc solution to serve as green technology for the purification of mine tailings. Chemosphere 2020, 249, 126227. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hu, C.; Li, B.; Ding, D.; Zhao, Z.; Fan, T.; Li, Z. Subsurface drip irrigation reduces cadmium accumulation of pepper (Capsicum annuum L.) plants in upland soil. Sci. Total Environ. 2021, 755 Pt 2, 142650. [Google Scholar] [CrossRef]
- Xu, J.; Zeng, M.; Wang, G.; Zhou, H.; Yang, W.; Gu, J.; Liao, B. Remediation of paddy soil complexly polluted with cadmium and arsenic using 2 combined amendments. Acta Sci. Circumstantiae 2018, 38, 2008–2013. [Google Scholar]
- Lu, H.-L.; Nkoh, J.N.; Biswash, M.R.; Hua, H.; Dong, G.; Li, J.-Y.; Xu, R.-K. Effects of surface charge and chemical forms of manganese(II) on rice roots on manganese absorption by different rice varieties. Ecotoxicol. Environ. Saf. 2020, 207, 111224. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xiong, J.; Ma, Q.; Zhang, D.; He, Z.; Wang, J. Effect of different amendments on growth and heavy metal accumulation in Vicia villosa Roth varglabrescens cv Yunguangzao in soils polluted with lead/zinc mine tailings. Chin. J. Eco-Agric. 2010, 18, 158–163. [Google Scholar] [CrossRef]
- Martins, G.C.; Penido, E.S.; Alvarenga, I.F.S.; Teodoro, J.C.; Bianchi, M.L.; Guilherme, L.R.G. Amending potential of organic and industrial by-products applied to heavy metal-rich mining soils. Ecotoxicol. Environ. Saf. 2018, 162, 581–590. [Google Scholar] [CrossRef]
- Zhao, X.; Jiang, T.; Du, B. Effect of organic matter and calcium carbonate on behaviors of cadmium adsorption-desorption on/from purple paddy soils. Chemosphere 2014, 99, 41–48. [Google Scholar] [CrossRef]
- Abu-El-Halawa, D.R.; Rami, Q. Efficiency of removal of lead, cadmium, copper and zinc from aqueous solutions using six common types of plant leaves. J. Appl. Sci. 2003, 3, 79–84. [Google Scholar] [CrossRef]
- Galende, M.A.; Becerril, J.M.; Barrutia, O.; Artetxe, U.; Garbisu, C.; Hernández, A. Field assessment of the effectiveness of organic amendments for aided phytostabilization of a Pb–Zn contaminated mine soil. J. Geochem. Explor. 2014, 145, 181–189. [Google Scholar] [CrossRef]
- Kabas, S.; Faz, A.; Acosta, J.A.; Zornoza, R.; Martínez-Martínez, S.; Carmona, D.M.; Bech, J. Effect of marble waste and pig slurry on the growth of native vegetation and heavy metal mobility in a mine tailing pond. J. Geochem. Explor. 2012, 123, 69–76. [Google Scholar] [CrossRef]
- Li, J.; Zhou, X.; Yan, J.; Li, H.; He, J. Effects of regenerating vegetation on soil enzyme activity and microbial structure in reclaimed soils on a surface coal mine site. Appl. Soil Ecol. 2015, 87, 56–62. [Google Scholar] [CrossRef]
- Galende, M.A.; Becerril, J.M.; Gómez-Sagasti, M.T.; Barrutia, O.; Epelde, L.; Garbisu, C.; Hernández, A. Chemical stabilization of metal-contaminated mine soil: Early short-term soil-amendment interactions and their effects on biological and chemical parameters. Water Air Soil Pollut. 2014, 225, 1863. [Google Scholar] [CrossRef]
- Bolan, N.; Kunhikrishnan, A.; Thangarajan, R.; Kumpiene, J.; Park, J.H.; Makino, T.; Kirkham, M.; Scheckel, K. Remediation of heavy metal(loid)s contaminated soils–To mobilize or to immobilize? J. Hazard. Mater. 2013, 266, 141–166. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.Z.; Yang, S.X.; Mei, L.F.; Cao, J.B.; Peng, Q.J.; University, J. Effects of three industrial organic wastes as amendments on plant growth and the biochemical properties of a Pb/Zn mine tailings. Environ. Sci. 2016, 37, 301–308. [Google Scholar]
- Xie, T.; Chen, Y.; Su, R.; Liu, H.; Yao, H. Mechanism of lead-zinc enrichment and resistance of spent mushroom compost to lead-zinc slag in Koelreutie paniculata. Environ. Sci. 2022. [Google Scholar] [CrossRef]
- He, L.; Chen, Y.; Su, R.; Cai, B.; Wang, J.; Zhang, A.; Zhu, H. Mechanism of strengthening manganese slag by composite modifier. J. Cent. South Univ. For. Technol. 2022, 42, 170–179. (In Chinese) [Google Scholar]







| Treatment Group | The Ratio of the Substrate |
|---|---|
| Control group CK | 100% tailing + 0.66 kg calcium magnesium phosphate fertilizer |
| Treatment group C1 | 95% tailing + 5% calcium carbonate + 0.66 kg calcium magnesium phosphate fertilizer |
| Treatment group C2 | 90% tailing + 10% calcium carbonate + 0.66 kg calcium magnesium phosphate fertilizer |
| Treatment group C3 | 85% tailing + 15% calcium carbonate + 0.66 kg calcium magnesium phosphate fertilizer |
| Treatment group M1 | 90% tailing + 10% mushroom residue + 0.66 kg calcium magnesium phosphate fertilizer |
| Treatment group M2 | 80% tailing + 20% mushroom residue + 0.66 kg calcium magnesium phosphate fertilizer |
| Treatment group M3 | 70% tailing + 30% mushroom residue + 0.66 kg calcium magnesium phosphate fertilizer |
| Substrates and Criteria | pH | Organic Matter (mg/kg) | Heavy Metal Content (mg/kg) | |||
|---|---|---|---|---|---|---|
| Pb | Zn | Cu | Cd | |||
| CK | 7.21 | 25.95 | 4297.08 | 1704.92 | 163.50 | 43.83 |
| C1 | 7.29 | 22.89 | 4135.58 | 1651.83 | 161.75 | 42.83 |
| C2 | 7.31 | 22.69 | 3916.42 | 1573.25 | 153.00 | 40.00 |
| C3 | 7.58 | 21.42 | 3894.17 | 1400.42 | 150.17 | 33.42 |
| M1 | 7.24 | 31.71 | 4011.25 | 1613.83 | 158.17 | 39.50 |
| M2 | 7.18 | 33.80 | 3702.25 | 1448.67 | 153.92 | 29.17 |
| M3 | 6.95 | 37.66 | 3311.50 | 1398.33 | 143.33 | 28.92 |
| National Ⅱ level standard | / | / | ≤300 | ≤250 | ≤100 | ≤0.3 |
| Chinese soil mean value | / | / | 26.0 | 74.2 | 22.6 | 0.097 |
| Soil background value of Hunan province | / | / | 26.3 | 90.0 | 25.0 | 0.081 |
| Improved Gradient | Root Biomass (g/Pot) | Stem Biomass (g/Pot) | Leaf Biomass (g/Pot) | The Whole Plant Biomass (g/Pot) |
|---|---|---|---|---|
| CK | 30.06 ± 5.20 c | 47.89 ± 5.99 d | 30.86 ± 1.71 c | 108.82 ± 3.76 c |
| C1 | 48.89 ± 7.44 bc | 49.56 ± 4.14 d | 33.36 ± 2.20 c | 131.83 ± 13.68 c |
| C2 | 54.09 ± 14.40 b | 55.06 ± 20.09 cd | 33.86 ± 2.86 c | 143.02 ± 34.12 c |
| C3 | 53.29 ± 6.33 b | 56.73 ± 10.53 cd | 42.20 ± 6.65 c | 152.22 ± 23.32 c |
| M1 | 56.30 ± 4.27 b | 96.33 ± 24.25 bc | 100.56 ± 31.54 b | 253.20 ± 58.85 b |
| M2 | 58.40 ± 10.13 b | 115.70 ± 18.70 b | 126.03 ± 12.47 b | 300.13 ± 36.26 b |
| M3 | 98.33 ± 10.60 a | 177.66 ± 24.76 a | 200.80 ± 23.75 a | 476.80 ± 48.11 a |
| Treatment Group | Heavy Metal Content in Each Organ (mg/kg) | |||||
|---|---|---|---|---|---|---|
| Pb | Zn | |||||
| Root | Stem | Leaf | Root | Stem | Leaf | |
| CK | 328.21 ± 6.35 a | 66.82 ± 12.15 bc | 16.67 ± 0.54 c | 487.08 ± 32.07 a | 88.58 ± 19.64 a | 51.12 ± 2.21 a |
| C1 | 113.44 ± 14.69 d | 88.92 ± 6.438 ab | 29.71 ± 3.91 a | 189.83 ± 28.93 f | 84.29 ± 15.48 ab | 27.5 ± 7.43 cd |
| C2 | 154.37 ± 11.82 c | 104.20 ± 15.21 a | 25.68 ± 2.62 ab | 224.83 ± 22.91 ef | 53.75 ± 12.58 bc | 26.33 ± 5.50 cd |
| C3 | 201.87 ± 17.54 b | 112.68 ± 19.35 a | 23.80 ± 1.15 b | 281.83 ± 38.86 de | 50.5 ± 18.01 bc | 25.29 ± 5.05 d |
| M1 | 189.01 ± 17.82 bc | 43.66 ± 7.11 cd | 8.87 ± 0.42 d | 322.58 ± 10.66 cd | 67.33 ± 3.88 abc | 48.41 ± 2.02 ab |
| M2 | 197.28 ± 21.38 b | 27.49 ± 5.14 de | 6.66 ± 0.99 d | 378.08 ± 26.12 bc | 55.87 ± 4.18 abc | 41.70 ± 1.58 ab |
| M3 | 212.27 ± 18.20 b | 15.14 ± 2.52 e | 5.86 ± 0.92 d | 398.33 ± 5.29 b | 49.79 ± 2.75 c | 38.04 ± 6.07 bc |
| Treatment Group | Heavy Metal Accumulation in Each Organ (mg/Pot) | |||||
|---|---|---|---|---|---|---|
| Pb | Zn | |||||
| Root | Stem | Leaf | Root | Stem | Leaf | |
| CK | 3.19 ± 0.84 ab | 1.08 ± 0.34 a | 0.17 ± 0.00 b | 4.72 ± 1.25 c | 1.44 ± 0.51 a | 0.53 ± 0.06 c |
| C1 | 1.98 ± 0.43 c | 1.49 ± 0.07 a | 0.32 ± 0.03 ab | 3.30 ± 0.57 c | 1.41 ± 0.25 a | 0.30 ± 0.09 c |
| C2 | 2.81 ± 0.80 bc | 1.90 ± 0.82 a | 0.29 ± 0.04 ab | 4.16 ± 1.56 c | 0.91 ± 0.10 a | 0.30 ± 0.08 c |
| C3 | 3.61 ± 0.71 bc | 2.13 ± 0.06 a | 0.33 ± 0.04 ab | 5.06 ± 1.25 c | 0.94 ± 0.26 a | 0.36 ± 0.13 c |
| M1 | 3.45 ± 0.83 bc | 1.50 ± 0.10 a | 0.26 ± 0.07 ab | 5.86 ± 1.11 c | 2.34 ± 0.18 b | 1.45 ± 0.42 b |
| M2 | 4.41 ± 0.38 b | 1.34 ± 0.45 a | 0.28 ± 0.07 ab | 8.46 ± 0.43 b | 2.66 ± 0.52 b | 1.82 ± 0.50 ab |
| M3 | 8.07 ± 0.94 a | 1.14 ± 0.35 a | 0.40 ± 0.07 a | 15.12 ± 0.45 a | 3.70 ± 0.45 a | 2.63 ± 0.64 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, R.; Ou, Q.; Wang, H.; Luo, Y.; Dai, X.; Wang, Y.; Chen, Y.; Shi, L. Comparison of Phytoremediation Potential of Nerium indicum with Inorganic Modifier Calcium Carbonate and Organic Modifier Mushroom Residue to Lead–Zinc Tailings. Int. J. Environ. Res. Public Health 2022, 19, 10353. https://doi.org/10.3390/ijerph191610353
Su R, Ou Q, Wang H, Luo Y, Dai X, Wang Y, Chen Y, Shi L. Comparison of Phytoremediation Potential of Nerium indicum with Inorganic Modifier Calcium Carbonate and Organic Modifier Mushroom Residue to Lead–Zinc Tailings. International Journal of Environmental Research and Public Health. 2022; 19(16):10353. https://doi.org/10.3390/ijerph191610353
Chicago/Turabian StyleSu, Rongkui, Qiqi Ou, Hanqing Wang, Yiting Luo, Xiangrong Dai, Yangyang Wang, Yonghua Chen, and Lei Shi. 2022. "Comparison of Phytoremediation Potential of Nerium indicum with Inorganic Modifier Calcium Carbonate and Organic Modifier Mushroom Residue to Lead–Zinc Tailings" International Journal of Environmental Research and Public Health 19, no. 16: 10353. https://doi.org/10.3390/ijerph191610353