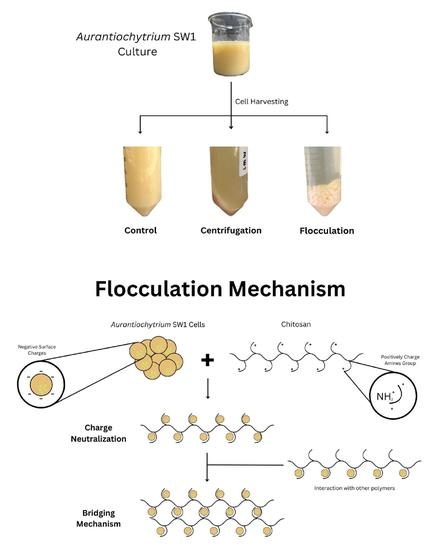
Harvesting Aurantiochytrium sp. SW1 via Flocculation Using Chitosan: Effects of Flocculation Parameters on Flocculation Efficiency and Zeta Potential
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Chitosan Concentration
2.2. Effect of Molecular Weight
2.3. Effect of pH
2.4. Culture Age and Cell Density
3. Materials and Methods
3.1. Aurantiochytrium sp. SW1 Cultivation
3.2. Flocculation Experiment
3.2.1. Flocculant Preparation
3.2.2. Flocculation Optimization for Harvesting Aurantiochytrium sp. SW1
3.3. Zeta Potential Analysis
3.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pratiwy, F.M.; Pratiwi, D.Y. The Potentiality of Microalgae as a Source of DHA and EPA for Aquaculture Feed: A Review. Int. J. Fish. Aquat. Stud. 2020, 8, 39–41. [Google Scholar]
- Sun, G.Y.; Simonyi, A.; Fritsche, K.L.; Chuang, D.Y.; Hannink, M.; Gu, Z.; Greenlief, C.M.; Yao, J.K.; Lee, J.C.; Beversdorf, D.Q. Docosahexaenoic Acid (DHA): An Essential Nutrient and a Nutraceutical for Brain Health and Diseases. Prostaglandins Leukot. Essent. Fat. Acids 2018, 136, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Wanasundara, N.U. Omega-3 fatty acid concentrates: Nutritional aspects and production technologies. Trends Food Sci. Technol. 1998, 9, 230–240. [Google Scholar] [CrossRef]
- Ghasemi Fard, S.; Wang, F.; Sinclair, A.J.; Elliott, G.; Turchini, G.M. How Does High DHA Fish Oil Affect Health? A Systematic Review of Evidence. Crit. Rev. Food Sci. Nutr. 2019, 59, 1684–1727. [Google Scholar] [CrossRef] [PubMed]
- Oliver, L.; Dietrich, T.; Marañón, I.; Villarán, M.C.; Barrio, R.J. Producing Omega-3 Polyunsaturated Fatty Acids: A Review of Sustainable Sources and Future Trends for the EPA and DHA Market. Resources 2020, 9, 148. [Google Scholar] [CrossRef]
- Béligon, V.; Christophe, G.; Fontanille, P.; Larroche, C. Microbial Lipids as Potential Source to Food Supplements. Curr. Opin. Food Sci. 2016, 7, 35–42. [Google Scholar] [CrossRef]
- Patel, A.; Rova, U.; Christakopoulos, P.; Matsakas, L. Simultaneous Production of DHA and Squalene from Aurantiochytrium sp. Grown on Forest Biomass Hydrolysates. Biotechnol. Biofuels 2019, 12, 255. [Google Scholar] [CrossRef]
- Zhang, A.; Xie, Y.; He, Y.; Wang, W.; Sen, B.; Wang, G. Bio-Based Squalene Production by Aurantiochytrium sp. through Optimization of Culture Conditions, and Elucidation of the Putative Biosynthetic Pathway Genes. Bioresour Technol. 2019, 287, 121415. [Google Scholar] [CrossRef]
- Bleisch, R.; Freitag, L.; Ihadjadene, Y.; Sprenger, U.; Steingröwer, J.; Walther, T.; Krujatz, F. Strain Development in Microalgal Biotechnology—Random Mutagenesis Techniques. Life 2022, 12, 961. [Google Scholar] [CrossRef]
- Manikan, V.; Nazir, Y.; Hamid, A.A. Two-Level Factorial Analysis of the Effect of Fructose on DHA Biosynthetic Capacity of Aurantiochytrium sp. SW1. Heliyon 2021, 7, e06085. [Google Scholar] [CrossRef]
- Park, S.; Nguyen, T.H.T.; Jin, E.S. Improving Lipid Production by Strain Development in Microalgae: Strategies, Challenges and Perspectives. Bioresour. Technol. 2019, 292, 121953. [Google Scholar] [CrossRef] [PubMed]
- Safavi, M.; Jafari Olia, M.S.; Abolhasani, M.H.; Amini, M.; Kianirad, M. Optimization of the Culture Medium and Characterization of Antioxidant Compounds of a Marine Isolated Microalga as a Promising Source in Aquaculture Feed. Biocatal. Agric. Biotechnol. 2021, 35, 102098. [Google Scholar] [CrossRef]
- González-Fernández, C.; Ballesteros, M. Microalgae Autoflocculation: An Alternative to High-Energy Consuming Harvesting Methods. J. Appl. Phycol. 2013, 25, 991–999. [Google Scholar] [CrossRef]
- Barros, A.I.; Gonçalves, A.L.; Simões, M.; Pires, J.C.M. Harvesting Techniques Applied to Microalgae: A Review. Renew. Sustain. Energy Rev. 2015, 41, 1489–1500. [Google Scholar] [CrossRef]
- Uduman, N.; Qi, Y.; Danquah, M.K.; Forde, G.M.; Hoadley, A. Dewatering of Microalgal Cultures: A Major Bottleneck to Algae-Based Fuels. J. Renew. Sustain. Energy 2010, 2, 012701. [Google Scholar] [CrossRef]
- Najjar, Y.S.H.; Abu-Shamleh, A. Harvesting of Microalgae by Centrifugation for Biodiesel Production: A Review. Algal Res. 2020, 51, 102046. [Google Scholar] [CrossRef]
- Wong, M.K.M.; Tsui, C.K.M.; Au, D.W.T.; Vrijmoed, L.L.P. Docosahexaenoic Acid Production and Ultrastructure of the Thraustochytrid Aurantiochytrium mangrovei MP2 under High Glucose Concentrations. Mycoscience 2008, 49, 266–270. [Google Scholar] [CrossRef]
- Kim, K.; Shin, H.; Moon, M.; Ryu, B.G.; Han, J.I.; Yang, J.W.; Chang, Y.K. Evaluation of Various Harvesting Methods for High-Density Microalgae, Aurantiochytrium sp. KRS101. Bioresour. Technol. 2015, 198, 828–835. [Google Scholar] [CrossRef]
- Patel, A.; Liefeldt, S.; Rova, U.; Christakopoulos, P.; Matsakas, L. Co-Production of DHA and Squalene by Thraustochytrid from Forest Biomass. Sci. Rep. 2020, 10, 1992. [Google Scholar] [CrossRef]
- Mokhtar, N.; Chang, L.S.; Soon, Y.; Wan Mustapha, W.A.; Sofian-Seng, N.S.; Rahman, H.A.; Mohd Razali, N.S.; Shuib, S.; Abdul Hamid, A.; Lim, S.J. Harvesting Aurantiochytrium sp. SW1 Using Organic Flocculants and Characteristics of the Extracted Oil. Algal Res. 2021, 54, 102211–102219. [Google Scholar] [CrossRef]
- Hadiyanto, H.; Widayat, W.; Christwardana, M.; Pratiwi, M.E. The Flocculation Process of Chlorella sp. Using Chitosan as a Bio-Flocculant: Optimization of Operating Conditions by Response Surface Methodology. Curr. Res. Green Sustain. Chem. 2022, 5, 100291–100302. [Google Scholar] [CrossRef]
- Blockx, J.; Verfaillie, A.; Thielemans, W.; Muylaert, K. Unravelling the Mechanism of Chitosan-Driven Flocculation of Microalgae in Seawater as a Function of pH. ACS Sustain. Chem. Eng. 2018, 6, 11273–11279. [Google Scholar] [CrossRef]
- Renault, F.; Sancey, B.; Badot, P.M.; Crini, G. Chitosan for Coagulation/Flocculation Processes—An Eco-Friendly Approach. Eur. Polym. J. 2009, 45, 1337–1348. [Google Scholar] [CrossRef]
- Akış, S.; Özçimen, D. Optimization of pH Induced Flocculation of Marine and Freshwater Microalgae via Central Composite Design. Biotechnol. Prog. 2019, 35, 4–9. [Google Scholar] [CrossRef]
- Pandey, A.; Pathak, V.V.; Kothari, R.; Black, P.N.; Tyagi, V.V. Experimental Studies on Zeta Potential of Flocculants for Harvesting of Algae. J. Environ. Manag. 2019, 231, 562–569. [Google Scholar] [CrossRef]
- Kothari, R.; Pathak, V.V.; Pandey, A.; Ahmad, S.; Srivastava, C.; Tyagi, V.V. A Novel Method to Harvest Chlorella Sp. via Low Cost Bioflocculant: Influence of Temperature with Kinetic and Thermodynamic Functions. Bioresour. Technol. 2017, 225, 84–89. [Google Scholar] [CrossRef]
- Shen, Y.; Cui, Y.; Yuan, W. Flocculation Optimization of Microalga Nannochloropsis oculata. Appl. Biochem. Biotechnol. 2013, 169, 2049–2063. [Google Scholar] [CrossRef]
- Soomro, R.R.; Ndikubwimana, T.; Zeng, X.; Lu, Y.; Lin, L.; Danquah, M.K. Development of a Two-Stage Microalgae Dewatering Process—A Life Cycle Assessment Approach. Front. Plant. Sci. 2016, 7, 113. [Google Scholar] [CrossRef]
- Alam, M.A.; Wang, Z.; Yuan, Z. Generation and Harvesting of Microalgae Biomass for Biofuel Production. In Prospects and Challenges in Algal Biotechnology; Springer: Singapore, 2017; pp. 89–111. ISBN 9789811019500. [Google Scholar]
- Manikan, V.; Nazir, M.Y.M.; Kalil, M.S.; Isa, M.H.M.; Kader, A.J.A.; Yusoff, W.M.W.; Hamid, A.A. A New Strain of Docosahexaenoic Acid Producing Microalga from Malaysian Coastal Waters. Algal Res. 2015, 9, 40–47. [Google Scholar] [CrossRef]
- Endrawati, H.; Widianingsih, W.; Nuraini, R.A.T.; Hartati, R.; Redjeki, S.; Riniatsih, I.; Mahendrajaya, R.T. The Effect of Chitosan Concentration on Flocculation Efficiency Microalgae Porphyridium Cruentum (Rhodhophyta). In Proceedings of the IOP Conference Series: Earth and Environmental Science, 2021 4th International Symposium on Marine and Fisheries Research, Yogyakarta, Indonesia, 28–29 July 2021; IOP Publishing: Bristol, UK, 2021; Volume 919, p. 012052. [Google Scholar] [CrossRef]
- Bharti, S. A Critical Review on Flocculants and Flocculation. Non-Met. Mater. Sci. 2019, 1, 11–21. [Google Scholar] [CrossRef]
- Zhu, L.; Li, Z.; Hiltunen, E. Microalgae Chlorella vulgaris Biomass Harvesting by Natural Flocculant: Effects on Biomass Sedimentation, Spent Medium Recycling and Lipid Extraction. Biotechnol. Biofuels 2018, 11, 183. [Google Scholar] [CrossRef] [PubMed]
- Billuri, M.; Bonner, J.S.; Fuller, C.B.; Islam, M.S. Impact of Natural Cationic Polymers on Charge and Clarification of Microalgae Suspensions. Environ. Eng. Sci. 2015, 32, 212–221. [Google Scholar] [CrossRef]
- Gerde, J.A.; Yao, L.; Lio, J.Y.; Wen, Z.; Wang, T. Microalgae Flocculation: Impact of Flocculant Type, Algae Species and Cell Concentration. Algal Res. 2014, 3, 30–35. [Google Scholar] [CrossRef]
- Low, Y.J.; Lau, S.W. Effective Flocculation of Chlorella vulgaris Using Chitosan with Zeta Potential Measurement. IOP Conf. Ser. Mater. Sci. Eng. 2017, 206, 012073. [Google Scholar] [CrossRef]
- Shatat, R. The Effect of Molecular Weight and Charge Density on Floc Size Distribution of Palm Oil Mill Effluent Flocculated with Cationic Polyelectrolytes. J. Basic Appl. Sci. 2008, 4, 95–103. [Google Scholar]
- Kirnev, P.C.S.; de Carvalho, J.C.; Miyaoka, J.T.; Cartas, L.C.; Vandenberghe, L.P.S.; Soccol, C.R. Harvesting Neochloris oleoabundans Using Commercial Organic Flocculants. J. Appl. Phycol. 2018, 30, 2317–2324. [Google Scholar] [CrossRef]
- Yaneva, Z.; Ivanova, D.; Nikolova, N.; Tzanova, M. The 21st Century Revival of Chitosan in Service to Bio-Organic Chemistry. Biotechnol. Biotechnol. Equip. 2020, 34, 221–237. [Google Scholar] [CrossRef]
- Jusoh, H.H.W.; Yunos, F.H.M.; Nasir, N.M.; Hamzah, S.; Jusoh, A. Utilization of Auto-Flocculate of Microalgae, Scededesmus sp. for Harvesting of Freshwater Microalgae, Chlorella sp. Biomass. J. Sustain. Sci. Manag. 2019, 14, 83–89. [Google Scholar]
- Hao, W.; Yanpeng, L.; Zhou, S.; Xiangying, R.; Wenjun, Z.; Jun, L. Surface Characteristics of Microalgae and Their Effects on Harvesting Performance by Air Flotation. Int. J. Agric. Biol. Eng. 2017, 10, 125–133. [Google Scholar]
- Garzon-Sanabria, A.J.; Ramirez-Caballero, S.S.; Moss, F.E.P.; Nikolov, Z.L. Effect of Algogenic Organic Matter (AOM) and Sodium Chloride on Nannochloropsis salina Flocculation Efficiency. Bioresour. Technol. 2013, 143, 231–237. [Google Scholar] [CrossRef]
- Nazir, Y.; Shuib, S.; Kalil, M.S.; Song, Y.; Hamid, A.A. Optimization of Culture Conditions for Enhanced Growth, Lipid and Docosahexaenoic Acid (DHA) Production of Aurantiochytrium SW1 by Response Surface Methodology. Sci. Rep. 2018, 8, 8909. [Google Scholar] [CrossRef] [PubMed]
- Vu, H.P.; Nguyen, L.N.; Emmerton, B.; Wang, Q.; Ralph, P.J.; Nghiem, L.D. Factors Governing Microalgae Harvesting Efficiency by Flocculation Using Cationic Polymers. Bioresour. Technol. 2021, 340, 125669. [Google Scholar] [CrossRef] [PubMed]
- Roselet, F.; Vandamme, D.; Roselet, M.; Muylaert, K.; Abreu, P.C. Effects of pH, Salinity, Biomass Concentration, and Algal Organic Matter on Flocculant Efficiency of Synthetic Versus Natural Polymers for Harvesting Microalgae Biomass. Bioenergy Res. 2017, 10, 427–437. [Google Scholar] [CrossRef]





| Optimal Concentration | Flocculation Capacity (g Chitosan/g Biomass) | Species | Biomass, g/L | Flocculation Efficiencies, % | References |
|---|---|---|---|---|---|
| 0.5 g/L | 0.03 | Aurantiochytrium sp. SW1 | 15.0 ± 2.83 | 95.58 ± 1.35 | This study |
| 0.25 g/L | 0.2 | Chlorella vulgaris | 1.2 | 91.9 ± 2.6 | [33] |
| 0.05 g/L | 0.1 | Chlorella vulgaris | 0.5 | 92.3 ± 2.5 | [33] |
| 0.05 g/g | 0.05 | Nannochloropsis salina | 0.95 | 97 | [34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamri, N.; Suleiman, N.N.; Mohd Johar, N.; Mohd Noor, N.S.; Ang, W.L.; Mohd Yasin, N.H.; Nazir, Y.; Abdul Hamid, A. Harvesting Aurantiochytrium sp. SW1 via Flocculation Using Chitosan: Effects of Flocculation Parameters on Flocculation Efficiency and Zeta Potential. Mar. Drugs 2023, 21, 251. https://doi.org/10.3390/md21040251
Zamri N, Suleiman NN, Mohd Johar N, Mohd Noor NS, Ang WL, Mohd Yasin NH, Nazir Y, Abdul Hamid A. Harvesting Aurantiochytrium sp. SW1 via Flocculation Using Chitosan: Effects of Flocculation Parameters on Flocculation Efficiency and Zeta Potential. Marine Drugs. 2023; 21(4):251. https://doi.org/10.3390/md21040251
Chicago/Turabian StyleZamri, Nadzirul, Nurul Nabila Suleiman, Norsyaqira Mohd Johar, Nur Syahidah Mohd Noor, Wei Lun Ang, Nazlina Haiza Mohd Yasin, Yusuf Nazir, and Aidil Abdul Hamid. 2023. "Harvesting Aurantiochytrium sp. SW1 via Flocculation Using Chitosan: Effects of Flocculation Parameters on Flocculation Efficiency and Zeta Potential" Marine Drugs 21, no. 4: 251. https://doi.org/10.3390/md21040251