Bioactive Oxylipins Profile in Marine Microalgae
Abstract
:1. Introduction
2. Results
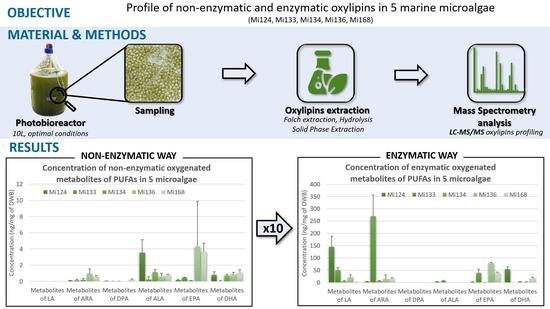
2.1. Omega-6 Oxidative Derivatives in Microalgae
2.1.1. Derivatives of LA (C18:2 n-6)
2.1.2. Derivatives of ARA (C20:4 n-6)
- Enzymatic oxylipins profile
- Non-enzymatic oxylipins profile
2.1.3. Derivatives of Omega-6 DPA (C22:5 n-6)
2.2. Omega-3 Oxidative Derivatives in Microalgae
2.2.1. Derivatives of ALA
- Enzymatic Profile
- Non enzymatic profile
2.2.2. Derivatives of EPA
- Enzymatic profile
- Non-enzymatic profile
2.2.3. Derivatives of DHA
- Enzymatic profile
- Non-enzymatic profile
3. Discussion
4. Materials and Methods
4.1. The Microalgal Species and Culture Conditions
4.2. Non Enzymatic Oxylipins
4.2.1. Chemicals and Reagents
4.2.2. Algal Sample Preparation
4.2.3. Micro-LC-MS/MS Analysis
4.2.4. Validation of Sample Preparation
4.3. Enzymatic Oxylipins
4.3.1. Algal Sample Preparation
4.3.2. LC-MS/MS Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PUFA | polyunsaturated fatty acid |
| LA | linoleic acid |
| AA | arachidonic acid |
| DPA | docosapentanoic acid |
| ALA | alpha-linolenic acid |
| EPA | eicosapentaenoic acid |
| DHA | docosahexaenoic acid |
| OS | oxidative stress |
| NEO-PUFAs | non-enzymatic oxidized PUFAs |
| ROS | reactive oxygenated species |
| COX | cyclooxygenase |
| LOX | lipoxygenase |
| CYP | cytochrome |
| SPM | specialized proresolving mediators |
| DWB | dry-weight biomass |
| ME | matrix effect |
| ER | extraction recovery |
| PE | process efficiency |
| HODEs | hydroxy-octadecadienoic acids |
| LXs | lipoxins |
| LTs | leukotrienes |
| H-ETEs | hydroxy-eicosatetraenoic acids |
| EETs | epoxyeicosatrienoic acids |
| PGs | prostagladines |
| TXBs | thromboxanes |
| HEPE | hydroxyeicosapentaenoic acid |
| HDoHE | hydroxydocosahexaenoic acid |
| IsoPs | isoprostanes |
| NeuroPs | neuroprostanes |
| PhytoPs | Phytoprostanes |
| PhytoFs | phytofuranes |
References
- Cabioc’h, J.; Floc’h, J.-Y.; Le Toquin, A.; Boudouresque, C.-F.; Meinesz, A.; Verlaque, M. Guide Des Algues Des Mers d’Europe: Manche/Atlantique - Méditerranée: Bon Couverture rigide (1992)|Le-Livre. Available online: https://www.abebooks.fr/Guide-algues-mers-dEurope-Manche-Atlantique/30820668218/bd (accessed on 20 February 2023).
- Behrenfeld, M.J.; Halsey, K.H.; Milligan, A.J. Evolved Physiological Responses of Phytoplankton to Their Integrated Growth Environment. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 2687–2703. [Google Scholar] [CrossRef] [Green Version]
- Worden, A.Z.; Follows, M.J.; Giovannoni, S.J.; Wilken, S.; Zimmerman, A.E.; Keeling, P.J. Rethinking the Marine Carbon Cycle: Factoring in the Multifarious Lifestyles of Microbes. Science 2015, 347, 1257594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerlero De Rosbo, G.; Payen, L.; Bernard, O.; Mairet, F.; Grimaud, G.; Delclaux, E.; Rey, D.; Ras De Moncuit, M.; Houdon, A.-C.; Gagnepain, B.; et al. Algae Potential Resource Assessment for the Energy and Chemistry Sectors in France by 2030; Evaluation du Gisement Potentiel de Ressources Algales pour L’energie et la Chimie en France a Horizon 2030; U.S. Department of Energy: Washington, DC, USA; Office of Scientific and Technical Information: Oak Ridge, TN, USA, 2014.
- Novoveská, L.; Ross, M.E.; Stanley, M.S.; Pradelles, R.; Wasiolek, V.; Sassi, J.-F. Microalgal Carotenoids: A Review of Production, Current Markets, Regulations, and Future Direction. Mar. Drugs 2019, 17, 640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsoupras, A.; Brummell, C.; Kealy, C.; Vitkaitis, K.; Redfern, S.; Zabetakis, I. Cardio-Protective Properties and Health Benefits of Fish Lipid Bioactives; The Effects of Thermal Processing. Mar. Drugs 2022, 20, 187. [Google Scholar] [CrossRef]
- Conde, T.A.; Zabetakis, I.; Tsoupras, A.; Medina, I.; Costa, M.; Silva, J.; Neves, B.; Domingues, P.; Domingues, M.R. Microalgal Lipid Extracts Have Potential to Modulate the Inflammatory Response: A Critical Review. Int. J. Mol. Sci. 2021, 22, 9825. [Google Scholar] [CrossRef]
- Khozin-Goldberg, I.; Iskandarov, U.; Cohen, Z. LC-PUFA from Photosynthetic Microalgae: Occurrence, Biosynthesis, and Prospects in Biotechnology. Appl. Microbiol. Biotechnol. 2011, 91, 905–915. [Google Scholar] [CrossRef]
- Ruiz-López, N.; Sayanova, O.; Napier, J.A.; Haslam, R.P. Metabolic Engineering of the Omega-3 Long Chain Polyunsaturated Fatty Acid Biosynthetic Pathway into Transgenic Plants. J. Exp. Bot. 2012, 63, 2397–2410. [Google Scholar] [CrossRef]
- Sethi, S.; Eastman, A.Y.; Eaton, J.W. Inhibition of Phagocyte-Endothelium Interactions by Oxidized Fatty Acids: A Natural Anti-Inflammatory Mechanism? J. Lab. Clin. Med. 1996, 128, 27–38. [Google Scholar] [CrossRef]
- Song, W.-L.; Paschos, G.; Fries, S.; Reilly, M.P.; Yu, Y.; Rokach, J.; Chang, C.-T.; Patel, P.; Lawson, J.A.; FitzGerald, G.A. Novel Eicosapentaenoic Acid-Derived F3-Isoprostanes as Biomarkers of Lipid Peroxidation *. J. Biol. Chem. 2009, 284, 23636–23643. [Google Scholar] [CrossRef] [Green Version]
- Joumard-Cubizolles, L.; Lee, J.C.-Y.; Vigor, C.; Leung, H.H.; Bertrand-Michel, J.; Galano, J.-M.; Mazur, A.; Durand, T.; Gladine, C. Insight into the Contribution of Isoprostanoids to the Health Effects of Omega 3 PUFAs. Prostaglandins Other Lipid Mediat. 2017, 133, 111–122. [Google Scholar] [CrossRef]
- Jahn, U.; Galano, J.-M.; Durand, T. Beyond Prostaglandins—Chemistry and Biology of Cyclic Oxygenated Metabolites Formed by Free-Radical Pathways from Polyunsaturated Fatty Acids. Angew. Chem. Int. Ed. 2008, 47, 5894–5955. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.A.; Marnett, L.J. Introduction to Lipid Biochemistry, Metabolism, and Signaling. Chem. Rev. 2011, 111, 5817–5820. [Google Scholar] [CrossRef]
- Morrow, J.D.; Hill, K.E.; Burk, R.F.; Nammour, T.M.; Badr, K.F.; Roberts, L.J. A Series of Prostaglandin F2-like Compounds Are Produced in Vivo in Humans by a Non-Cyclooxygenase, Free Radical-Catalyzed Mechanism. Proc. Natl. Acad. Sci. USA 1990, 87, 9383–9387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galano, J.-M.; Lee, Y.Y.; Oger, C.; Vigor, C.; Vercauteren, J.; Durand, T.; Giera, M.; Lee, J.C.-Y. Isoprostanes, Neuroprostanes and Phytoprostanes: An Overview of 25 Years of Research in Chemistry and Biology. Prog. Lipid Res. 2017, 68, 83–108. [Google Scholar] [CrossRef] [PubMed]
- Taber, D.F.; Fessel, J.P.; Roberts, L.J. A Nomenclature System for Isofurans. Prostaglandins Other Lipid Mediat. 2004, 73, 47–50. [Google Scholar] [CrossRef]
- Andreou, A.; Brodhun, F.; Feussner, I. Biosynthesis of Oxylipins in Non-Mammals. Prog. Lipid Res. 2009, 48, 148–170. [Google Scholar] [CrossRef]
- Bannenberg, G.; Serhan, C.N. Specialized Pro-Resolving Lipid Mediators in the Inflammatory Response: An Update. Biochim. Et Biophys. Acta (BBA) - Mol. Cell Biol. Lipids 2010, 1801, 1260–1273. [Google Scholar] [CrossRef] [Green Version]
- Serhan, C.N.; Chiang, N.; Van Dyke, T.E. Resolving Inflammation: Dual Anti-Inflammatory and pro-Resolution Lipid Mediators. Nat. Rev. Immunol. 2008, 8, 349–361. [Google Scholar] [CrossRef] [Green Version]
- Altmann, R.; Hausmann, M.; Spöttl, T.; Gruber, M.; Bull, A.W.; Menzel, K.; Vogl, D.; Herfarth, H.; Schölmerich, J.; Falk, W.; et al. 13-Oxo-ODE Is an Endogenous Ligand for PPARγ in Human Colonic Epithelial Cells. Biochem. Pharmacol. 2007, 74, 612–622. [Google Scholar] [CrossRef]
- Serhan, C.N. Lipoxins and Aspirin-Triggered 15-Epi-Lipoxins Are the First Lipid Mediators of Endogenous Anti-Inflammation and Resolution. Prostaglandins Leukot. Essent. Fat. Acids 2005, 73, 141–162. [Google Scholar] [CrossRef]
- Balas, L.; Dey, S.K.; Béraud-Dufour, S.; Riechers, D.E.; Landau, O.A.; Bertrand-Michel, J.; Durand, T.; Blondeau, N. Linotrins: Omega-3 Oxylipins Featuring an E,Z,E Conjugated Triene Motif Are Present in the Plant Kingdom and Alleviate Inflammation in LPS-Challenged Microglial Cells. Eur. J. Med. Chem. 2022, 231, 114157. [Google Scholar] [CrossRef]
- Serhan, C.N.; Hong, S.; Gronert, K.; Colgan, S.P.; Devchand, P.R.; Mirick, G.; Moussignac, R.-L. Resolvins: A Family of Bioactive Products of Omega-3 Fatty Acid Transformation Circuits Initiated by Aspirin Treatment That Counter Proinflammation Signals. J. Exp. Med. 2002, 196, 1025–1037. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, P.K.; Marcheselli, V.L.; Serhan, C.N.; Bazan, N.G. Neuroprotectin D1: A Docosahexaenoic Acid-Derived Docosatriene Protects Human Retinal Pigment Epithelial Cells from Oxidative Stress. Proc. Natl. Acad. Sci. USA 2004, 101, 8491–8496. [Google Scholar] [CrossRef] [Green Version]
- Serhan, C.N.; Chiang, N.; Dalli, J. The Resolution Code of Acute Inflammation: Novel pro-Resolving Lipid Mediators in Resolution. Semin. Immunol. 2015, 27, 200–215. [Google Scholar] [CrossRef] [Green Version]
- Ji, R.-R.; Xu, Z.-Z.; Strichartz, G.; Serhan, C.N. Emerging Roles of Resolvins in the Resolution of Inflammation and Pain. Trends Neurosci. 2011, 34, 599–609. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, O.S.; Galano, J.-M.; Pavlickova, T.; Revol-Cavalier, J.; Vigor, C.; Lee, J.C.-Y.; Oger, C.; Durand, T. Moving Forward with Isoprostanes, Neuroprostanes and Phytoprostanes: Where Are We Now? Essays Biochem. 2020, 64, 463–484. [Google Scholar] [CrossRef]
- Praticò, D.; Smyth, E.M.; Violi, F.; FitzGerald, G.A. Local Amplification of Platelet Function by 8-Epi Prostaglandin F2α Is Not Mediated by Thromboxane Receptor Isoforms *. J. Biol. Chem. 1996, 271, 14916–14924. [Google Scholar] [CrossRef] [Green Version]
- Milne, G.L.; Dai, Q.; Roberts, L.J. The Isoprostanes—25 Years Later. Biochim. Et Biophys. Acta (BBA) - Mol. Cell Biol. Lipids 2015, 1851, 433–445. [Google Scholar] [CrossRef] [Green Version]
- Lappas, M.; Permezel, M.; Holdsworth, S.J.; Zanoni, G.; Porta, A.; Rice, G.E. Antiinflammatory Effects of the Cyclopentenone Isoprostane 15-A2-IsoP in Human Gestational Tissues. Free Radic. Biol. Med. 2007, 42, 1791–1796. [Google Scholar] [CrossRef]
- Musiek, E.S.; Gao, L.; Milne, G.L.; Han, W.; Everhart, M.B.; Wang, D.; Backlund, M.G.; DuBois, R.N.; Zanoni, G.; Vidari, G.; et al. Cyclopentenone Isoprostanes Inhibit the Inflammatory Response in Macrophages *. J. Biol. Chem. 2005, 280, 35562–35570. [Google Scholar] [CrossRef] [Green Version]
- Minghetti, L.; Salvi, R.; Lavinia Salvatori, M.; Ajmone-Cat, M.A.; De Nuccio, C.; Visentin, S.; Bultel-Poncé, V.; Oger, C.; Guy, A.; Galano, J.-M.; et al. Nonenzymatic Oxygenated Metabolites of α-Linolenic Acid B1- and L1-Phytoprostanes Protect Immature Neurons from Oxidant Injury and Promote Differentiation of Oligodendrocyte Progenitors through PPAR-γ Activation. Free Radic. Biol. Med. 2014, 73, 41–50. [Google Scholar] [CrossRef]
- González Roldán, N.; Engel, R.; Düpow, S.; Jakob, K.; Koops, F.; Orinska, Z.; Vigor, C.; Oger, C.; Galano, J.-M.; Durand, T.; et al. Lipid Mediators From Timothy Grass Pollen Contribute to the Effector Phase of Allergy and Prime Dendritic Cells for Glycolipid Presentation. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Galano, J.-M.; Mas, E.; Barden, A.; Mori, T.A.; Signorini, C.; De Felice, C.; Barrett, A.; Opere, C.; Pinot, E.; Schwedhelm, E.; et al. Isoprostanes and Neuroprostanes: Total Synthesis, Biological Activity and Biomarkers of Oxidative Stress in Humans. Prostaglandins Other Lipid Mediat. 2013, 107, 95–102. [Google Scholar] [CrossRef]
- Roy, J.; Oger, C.; Thireau, J.; Roussel, J.; Mercier-Touzet, O.; Faure, D.; Pinot, E.; Farah, C.; Taber, D.F.; Cristol, J.-P.; et al. Nonenzymatic Lipid Mediators, Neuroprostanes, Exert the Antiarrhythmic Properties of Docosahexaenoic Acid. Free Radic. Biol. Med. 2015, 86, 269–278. [Google Scholar] [CrossRef] [Green Version]
- Roy, J.; Fauconnier, J.; Oger, C.; Farah, C.; Angebault-Prouteau, C.; Thireau, J.; Bideaux, P.; Scheuermann, V.; Bultel-Poncé, V.; Demion, M.; et al. Non-Enzymatic Oxidized Metabolite of DHA, 4(RS)-4-F4t-Neuroprostane Protects the Heart against Reperfusion Injury. Free Radic. Biol. Med. 2017, 102, 229–239. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Galano, J.-M.; Leung, H.H.; Balas, L.; Oger, C.; Durand, T.; Lee, J.C.-Y. Nonenzymatic Oxygenated Metabolite of Docosahexaenoic Acid, 4(RS)-4-F4t-Neuroprostane, Acts as a Bioactive Lipid Molecule in Neuronal Cells. FEBS Lett. 2020, 594, 1797–1808. [Google Scholar] [CrossRef]
- Barbosa, M.; Collado-González, J.; Andrade, P.B.; Ferreres, F.; Valentão, P.; Galano, J.-M.; Durand, T.; Gil-Izquierdo, Á. Nonenzymatic α-Linolenic Acid Derivatives from the Sea: Macroalgae as Novel Sources of Phytoprostanes. J. Agric. Food Chem. 2015, 63, 6466–6474. [Google Scholar] [CrossRef]
- Vigor, C.; Reversat, G.; Rocher, A.; Oger, C.; Galano, J.-M.; Vercauteren, J.; Durand, T.; Tonon, T.; Leblanc, C.; Potin, P. Isoprostanoids Quantitative Profiling of Marine Red and Brown Macroalgae. Food Chem. 2018, 268, 452–462. [Google Scholar] [CrossRef] [Green Version]
- Gerwick, W.H. Structure and Biosynthesis of Marine Algal Oxylipins. Biochim. Et Biophys. Acta (BBA)-Lipids Lipid Metab. 1994, 1211, 243–255. [Google Scholar] [CrossRef]
- Kumari, P.; Reddy, R.; Jha, B. Quantification of Selected Endogenous Hydroxy-Oxylipins from Tropical Marine Macroalgae. Mar. Biotechnol (NY) 2014, 16, 74–87. [Google Scholar] [CrossRef]
- Barbosa, M.; Valentão, P.; Andrade, P.B. Biologically Active Oxylipins from Enzymatic and Nonenzymatic Routes in Macroalgae. Mar. Drugs 2016, 14, 23. [Google Scholar] [CrossRef] [Green Version]
- Lupette, J.; Jaussaud, A.; Vigor, C.; Oger, C.; Galano, J.-M.; Réversat, G.; Vercauteren, J.; Jouhet, J.; Durand, T.; Maréchal, E. Non-Enzymatic Synthesis of Bioactive Isoprostanoids in the Diatom Phaeodactylum Following Oxidative Stress. Plant Physiol. 2018, 178, 1344–1357. [Google Scholar] [CrossRef] [Green Version]
- Vigor, C.; Oger, C.; Reversat, G.; Rocher, A.; Zhou, B.; Linares-Maurizi, A.; Guy, A.; Bultel-Poncé, V.; Galano, J.-M.; Vercauteren, J.; et al. Isoprostanoid Profiling of Marine Microalgae. Biomolecules 2020, 10, 1073. [Google Scholar] [CrossRef]
- Parchmann, S.; Mueller, M.J. Evidence for the Formation of Dinor Isoprostanes E1from α-Linolenic Acid in Plants*. J. Biol. Chem. 1998, 273, 32650–32655. [Google Scholar] [CrossRef] [Green Version]
- Caussy, C.; Chuang, J.-C.; Billin, A.; Hu, T.; Wang, Y.; Subramanian, G.M.; Djedjos, C.S.; Myers, R.P.; Dennis, E.A.; Loomba, R. Plasma Eicosanoids as Noninvasive Biomarkers of Liver Fibrosis in Patients with Nonalcoholic Steatohepatitis. Ther. Adv. Gastroenterol. 2020, 13, 1756284820923904. [Google Scholar] [CrossRef]
- Serhan, C.N. Pro-Resolving Lipid Mediators Are Leads for Resolution Physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, S.M.; Domínguez-Perles, R.; Montoro-García, S.; Gabaldón, J.A.; Guy, A.; Durand, T.; Oger, C.; Ferreres, F.; Gil-Izquierdo, A. Bioavailable Phytoprostanes and Phytofurans from Gracilaria Longissima Have Anti-Inflammatory Effects in Endothelial Cells. Food Funct. 2020, 11, 5166–5178. [Google Scholar] [CrossRef] [PubMed]
- Medina, S.; Gil-Izquierdo, Á.; Durand, T.; Ferreres, F.; Domínguez-Perles, R. Structural/Functional Matches and Divergences of Phytoprostanes and Phytofurans with Bioactive Human Oxylipins. Antioxidants 2018, 7, 165. [Google Scholar] [CrossRef] [Green Version]
- Bosviel, R.; Joumard-Cubizolles, L.; Chinetti-Gbaguidi, G.; Bayle, D.; Copin, C.; Hennuyer, N.; Duplan, I.; Staels, B.; Zanoni, G.; Porta, A.; et al. DHA-Derived Oxylipins, Neuroprostanes and Protectins, Differentially and Dose-Dependently Modulate the Inflammatory Response in Human Macrophages: Putative Mechanisms through PPAR Activation. Free Radic. Biol. Med. 2017, 103, 146–154. [Google Scholar] [CrossRef]
- White, P.J.; Arita, M.; Taguchi, R.; Kang, J.X.; Marette, A. Transgenic Restoration of Long-Chain n-3 Fatty Acids in Insulin Target Tissues Improves Resolution Capacity and Alleviates Obesity-Linked Inflammation and Insulin Resistance in High-Fat–Fed Mice. Diabetes 2010, 59, 3066–3073. [Google Scholar] [CrossRef] [Green Version]
- Meyer, N.; Rettner, J.; Werner, M.; Werz, O.; Pohnert, G. Algal Oxylipins Mediate the Resistance of Diatoms against Algicidal Bacteria. Mar. Drugs 2018, 16, 486. [Google Scholar] [CrossRef] [Green Version]
- de los Reyes, C.; Ávila-Román, J.; Ortega, M.J.; de la Jara, A.; García-Mauriño, S.; Motilva, V.; Zubía, E. Oxylipins from the Microalgae Chlamydomonas Debaryana and Nannochloropsis Gaditana and Their Activity as TNF-α Inhibitors. Phytochemistry 2014, 102, 152–161. [Google Scholar] [CrossRef]
- Ávila-Román, J.; Talero, E.; Rodríguez-Luna, A.; García-Mauriño, S.; Motilva, V. Anti-Inflammatory Effects of an Oxylipin-Containing Lyophilised Biomass from a Microalga in a Murine Recurrent Colitis Model. Br. J. Nutr. 2016, 116, 2044–2052. [Google Scholar] [CrossRef] [Green Version]
- Ávila-Román, J.; Talero, E.; de los Reyes, C.; García-Mauriño, S.; Motilva, V. Microalgae-Derived Oxylipins Decrease Inflammatory Mediators by Regulating the Subcellular Location of NFκB and PPAR-γ. Pharmacol. Res. 2018, 128, 220–230. [Google Scholar] [CrossRef]
- Guillard, R.R.; Ryther, J.H. Studies of Marine Planktonic Diatoms. I. Cyclotella Nana Hustedt, and Detonula Confervacea (Cleve) Gran. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef]
- Matuszewski, B.K.; Constanzer, M.L.; Chavez-Eng, C.M. Strategies for the Assessment of Matrix Effect in Quantitative Bioanalytical Methods Based on HPLC−MS/MS. Anal. Chem. 2003, 75, 3019–3030. [Google Scholar] [CrossRef] [PubMed]
- Marchi, I.; Viette, V.; Badoud, F.; Fathi, M.; Saugy, M.; Rudaz, S.; Veuthey, J.-L. Characterization and Classification of Matrix Effects in Biological Samples Analyses. J. Chromatogr. A 2010, 1217, 4071–4078. [Google Scholar] [CrossRef] [Green Version]
- Badoud, F.; Grata, E.; Boccard, J.; Guillarme, D.; Veuthey, J.-L.; Rudaz, S.; Saugy, M. Quantification of Glucuronidated and Sulfated Steroids in Human Urine by Ultra-High Pressure Liquid Chromatography Quadrupole Time-of-Flight Mass Spectrometry. Anal. Bioanal. Chem. 2011, 400, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Le Faouder, P.; Baillif, V.; Spreadbury, I.; Motta, J.-P.; Rousset, P.; Chêne, G.; Guigné, C.; Tercé, F.; Vanner, S.; Vergnolle, N.; et al. LC–MS/MS Method for Rapid and Concomitant Quantification of pro-Inflammatory and pro-Resolving Polyunsaturated Fatty Acid Metabolites. J. Chromatogr. B 2013, 932, 123–133. [Google Scholar] [CrossRef]
- Galano, J.-M.; Roy, J.; Durand, T.; Lee, J.C.-Y.; Le Guennec, J.-Y.; Oger, C.; Demion, M. Biological Activities of Non-Enzymatic Oxygenated Metabolites of Polyunsaturated Fatty Acids (NEO-PUFAs) Derived from EPA and DHA: New Anti-Arrhythmic Compounds? Mol. Asp. Med. 2018, 64, 161–168. [Google Scholar] [CrossRef] [Green Version]
- Geng, X.; Galano, J.-M.; Oger, C.; Sun, G.Y.; Durand, T.; Lee, J.C. Neuroprotective Effects of DHA-Derived Peroxidation Product 4(RS)-4-F4t-Neuroprostane on Microglia. Free Radic. Biol. Med. 2022, 185, 1–5. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linares-Maurizi, A.; Reversat, G.; Awad, R.; Bultel-Poncé, V.; Oger, C.; Galano, J.-M.; Balas, L.; Durbec, A.; Bertrand-Michel, J.; Durand, T.; et al. Bioactive Oxylipins Profile in Marine Microalgae. Mar. Drugs 2023, 21, 136. https://doi.org/10.3390/md21030136
Linares-Maurizi A, Reversat G, Awad R, Bultel-Poncé V, Oger C, Galano J-M, Balas L, Durbec A, Bertrand-Michel J, Durand T, et al. Bioactive Oxylipins Profile in Marine Microalgae. Marine Drugs. 2023; 21(3):136. https://doi.org/10.3390/md21030136
Chicago/Turabian StyleLinares-Maurizi, Amandyne, Guillaume Reversat, Rana Awad, Valérie Bultel-Poncé, Camille Oger, Jean-Marie Galano, Laurence Balas, Anaelle Durbec, Justine Bertrand-Michel, Thierry Durand, and et al. 2023. "Bioactive Oxylipins Profile in Marine Microalgae" Marine Drugs 21, no. 3: 136. https://doi.org/10.3390/md21030136