Anti-Photoaging Effect of Hydrolysates from Pacific Whiting Skin via MAPK/AP-1, NF-κB, TGF-β/Smad, and Nrf-2/HO-1 Signaling Pathway in UVB-Induced Human Dermal Fibroblasts
Abstract
:1. Introduction
2. Results
2.1. Cell Viability of PWG
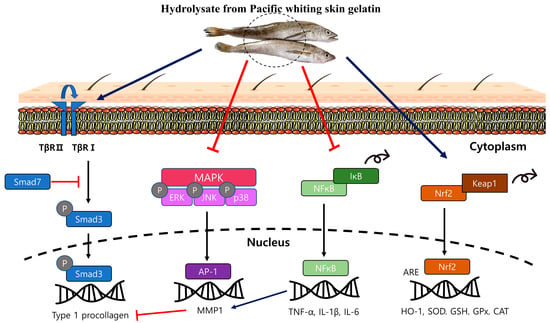
2.2. PWG Stimulates Type I Procollagen Synthesis and Inhibits MMP1 Synthesis in UVB-Irradiated HDFs
2.3. PWG Stimulates TGF-β/Smad Signaling Pathway in UVB-Irradiated HDFs
2.4. PWG Inhibits MAPK Signaling Pathway in UVB-Irradiated HDFs
2.5. PWG Inhibits AP-1 Expression in UVB-Irradiated HDFs
2.6. PWG Inhibits NF-κB Signaling Pathway in UVB-Irradiated HDFs
2.7. PWG Activates Nrf-2/HO-1 Signaling Pathway in UVB-Irradiated HDFs
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Pacific Whiting Skin Gelatin (PWG)
4.3. Cell Culture
4.4. UVB Irradiation and PWG Treatment
4.5. Cell Viability Assay
4.6. Determination of Antioxidant Enzyme Activity
4.7. RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Analysis
4.8. Western Blot Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hwang, K.-A.; Bo-Rim, Y.; Kyung-Chul, C. Molecular Mechanisms and in Vivo Mouse Models of Skin Aging Associated with Dermal Matrix Alterations. Lab. Anim. Res. 2011, 27, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naylor, C.E.; Watson, R.E.B.; Sherratt, M.J. Molecular Aspects of Skin Ageing. Maturitas 2011, 69, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Ananthaswamy, H.N. Toxic Effects of Ultraviolet Radiation on the Skin. Toxicol. Appl. Pharmacol. 2004, 195, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Scharffetter–Kochanek, K.; Brenneisen, P.; Wenk, J.; Herrmann, G.; Ma, W.; Kuhr, L.; Meewes, C.; Wlaschek, M. Photoaging of the Skin from Phenotype to Mechanisms. Exp. Gerontol. 2000, 35, 307–316. [Google Scholar] [CrossRef]
- Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Makrantonaki, E.; Zouboulis, C.C. Skin Anti-Aging Strategies. Dermato-endocrinology 2012, 3, 308–319. [Google Scholar] [CrossRef] [Green Version]
- Verma, R.P.; Hansch, C. Matrix Metalloproteinases (Mmps): Chemical–Biological Functions and (Q) Sars. Bioorg. Med. Chem. 2007, 6, 2223–2268. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Gaynor, R.B. Ikappab Kinases: Key Regulators of the Nf-Kappab Pathway. Trends Biochem. Sci. 2004, 29, 72–79. [Google Scholar] [CrossRef]
- Quan, T.; Shao, Y.; He, T.; Voorhees, J.J.; Fisher, G.J. Reduced Expression of Connective Tissue Growth Factor (Ctgf/Ccn2) Mediates Collagen Loss in Chronologically Aged Human Skin. J. Investig. Dermatol. 2010, 130, 415–424. [Google Scholar] [CrossRef] [Green Version]
- Varga, J.; Rosenbloom, J.; Jimenez, S.A. Transforming Growth Factor Β (Tgf Β) Causes a Persistent Increase in Steady-State Amounts of Type I and Type Iii Collagen and Fibronectin Mrnas in Normal Human Dermal Fibroblasts. Biochem. J. 1987, 247, 597–604. [Google Scholar] [CrossRef]
- He, T.; Quan, T.; Shao, Y.; Voorhees, J.J.; Fisher, G.J. Oxidative Exposure Impairs Tgf-Β Pathway Via Reduction of Type Ii Receptor and Smad3 in Human Skin Fibroblasts. Age 2014, 36, 1079–1094. [Google Scholar] [CrossRef] [Green Version]
- Brand, R.M.; Wipf, P.; Durham, A.; Epperly, M.W.; Greenberger, J.S.; Falo, L.D.J. Targeting Mitochondrial Oxidative Stress to Mitigate Uv-Induced Skin Damage. Front. Pharmacol. 2018, 9, 920. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Chiba, T.; Takahashi, S.; Ishii, T.; Igarashi, K.; Katoh, Y.; Oyake, T.; Hayashi, N.; Satoh, K.; Hatayama, I. An Nrf2/Small Maf Heterodimer Mediates the Induction of Phase Ii Detoxifying Enzyme Genes through Antioxidant Response Elements. Biochem. Biophys. Res. Commun. 1997, 236, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.D. Mechanistic Studies of the Nrf2-Keap1 Signaling Pathway. Drug Metab. Rev. 2006, 38, 769–789. [Google Scholar] [CrossRef] [PubMed]
- Mann, H.M. Achievements of the Pacific Whiting Conservation Cooperative: Rational Collaboration in a Sea of Irrational Competition. Case Stud. Fish. Self-Gov. 2008, 504, 425. [Google Scholar]
- Boran, G.; Regenstein, J.M. Fish Gelatin. Adv. Food Nutr. Res. 2010, 60, 119–143. [Google Scholar]
- Cheung, I.W.Y.; Cheung, L.K.Y.; Tan, N.Y.; Li-Chan, E.C.Y. The Role of Molecular Size in Antioxidant Activity of Peptide Fractions from Pacific Hake (Merluccius Productus) Hydrolysates. Food Chem. 2012, 134, 1297–1306. [Google Scholar] [CrossRef]
- Yaar, M.; Gilchrest, B.A. Photoageing: Mechanism, Prevention and Therapy. Br. J. Dermatol. 2007, 157, 874–887. [Google Scholar] [CrossRef]
- Rittié, L.; Fisher, G.J. Uv-Light-Induced Signal Cascades and Skin Aging. Ageing Res. Rev. 2002, 1, 705–720. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, H.E.; Cho, E.-B.; Kim, D.-H.; Kim, B.O.; Kang, I.K.; Jung, H.Y.; Cho, Y.J. Protective Effects of Galangin against Uvb Irradiation-Induced Photo-Aging in Ccd-986sk Human Skin Fibroblasts. Appl. Biol. Chem. 2019, 62, 1–8. [Google Scholar]
- Gao, W.; Wang, Y.S.; Hwang, E.; Lin, P.; Bae, J.; Seo, S.A.; Yan, Z.; Yi, T.H. Rubus Idaeus L. (Red Raspberry) Blocks Uvb-Induced Mmp Production and Promotes Type I Procollagen Synthesis Via Inhibition of Mapk/Ap-1, Nf-Κβ and Stimulation of Tgf-Β/Smad, Nrf2 in Normal Human Dermal Fibroblasts. J. Photochem. Photobiol. B Biol. 2018, 185, 241–253. [Google Scholar] [CrossRef]
- Cavinato, M.; Jansen-Dürr, P. Molecular Mechanisms of Uvb-Induced Senescence of Dermal Fibroblasts and Its Relevance for Photoaging of the Human Skin. Exp. Gerontol. 2017, 94, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Verrecchia, F.; Mauviel, A. Transforming Growth Factor-Β Signaling through the Smad Pathway: Role in Extracellular Matrix Gene Expression and Regulation. J. Investig. Dermatol. 2002, 118, 211–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, H.M.; Chen, C.W.; Lin, T.-Y.; Kuo, Y.-H. N-Phenethyl Caffeamide and Photodamage: Protecting Skin by Inhibiting Type I Procollagen Degradation and Stimulating Collagen Synthesis. Food Chem. Toxicol. 2014, 72, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Raman, M.; Chen, W.; Cobb, M.H. Differential Regulation and Properties of Mapks. Oncogene 2007, 26, 3100–3112. [Google Scholar] [CrossRef] [Green Version]
- Fisher, G.J.; Talwar, H.S.; Lin, J.; Lin, P.; McPhillips, F.; Wang, Z.Q.; Li, X.; Wan, Y.; Kang, S.; Voorhees, J.J. Retinoic Acid Inhibits Induction of C-Jun Protein by Ultraviolet Radiation That Occurs Subsequent to Activation of Mitogen-Activated Protein Kinase Pathways in Human Skin in Vivo. J. Clin. Investig. 1998, 101, 1432–1440. [Google Scholar] [CrossRef]
- Kim, A.L.; Labasi, J.M.; Zhu, Y.; Tang, X.; McClure, K.; Gabel, C.A.; Athar, M.; Bickers, D.R. Role of P38 Mapk in Uvb-Induced Inflammatory Responses in the Skin of Skh-1 Hairless Mice. J. Investig. Dermatol. 2005, 124, 1318–1325. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.M.; Kundu, J.K.; Shin, J.W.; Na, H.K.; Surh, Y.J. Docosahexaenoic Acid Inhibits Uvb-Induced Activation of Nf-Κb and Expression of Cox-2 and Nox-4 in Hr-1 Hairless Mouse Skin by Blocking Msk1 Signaling. Plos ONE 2011, 6, e28065. [Google Scholar] [CrossRef] [Green Version]
- Muthusamy, V.; Piva, T.J. The Uv Response of the Skin: A Review of the Mapk, Nfκb and Tnfα Signal Transduction Pathways. Arch. Dermatol. Res. 2010, 302, 5–17. [Google Scholar] [CrossRef]
- Lee, Y.-R.; Noh, E.M.; Jeong, E.Y.; Yun, S.K.; Jeong, Y.J.; Kim, J.-H.; Kwon, K.B.; Kim, B.S.; Lee, S.H.; Park, C.S.; et al. Cordycepin Inhibits Uvb-Induced Matrix Metalloproteinase Expression by Suppressing the Nf-Κb Pathway in Human Dermal Fibroblasts. Exp. Mol. Med. 2009, 41, 548–554. [Google Scholar] [CrossRef] [Green Version]
- Kondo, S. The Roles of Cytokines in Photoaging. J. Dermatol. Sci. 2000, 23, S30–S36. [Google Scholar] [CrossRef]
- Baugé, C.; Girard, N.; Leclercq, S.; Galéra, P.; Boumédiene, K. Regulatory Mechanism of Transforming Growth Factor Beta Receptor Type Ii Degradation by Interleukin-1 in Primary Chondrocytes. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2012, 5, 983–986. [Google Scholar] [CrossRef] [PubMed]
- Ichihashi, M.; Ando, H.; Yoshida, M.; Niki, Y.; Matsui, M. Photoaging of the Skin. Anti-Aging Med. 2009, 6, 46–59. [Google Scholar] [CrossRef] [Green Version]
- Solomon, H. The Nrf2/Ho-1 Axis as Targets for Flavanones: Neuroprotection by Pinocembrin, Naringenin, and Eriodictyol. Oxidative Med. Cell. Longev. 2019, 2019, 4724920. [Google Scholar] [CrossRef]
- Gęgotek, A.; Skrzydlewska, E. The Role of Transcription Factor Nrf2 in Skin Cells Metabolism. Arch. Dermatol. Res. 2015, 307, 385–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramachandran, S.; Prasad, N.R.; Karthikeyan, S. Sesamol Inhibits Uvb-Induced Ros Generation and Subsequent Oxidative Damage in Cultured Human Skin Dermal Fibroblasts. Arch. Dermatol. Res. 2010, 302, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-B.; Ding, B.X.; Guo, S.B.; Wang, Y.Z.; Han, Y.T.; Wang, Y.J. Protective Effect of Polypeptide from Chlamys Farreri on Mitochondria in Human Dermal Fibroblasts Irradiated by Ultraviolet B. Acta Pharmacol. Sin. 2003, 24, 692–696. [Google Scholar] [PubMed]
- Xian, D.; Xiong, X.; Xu, J.; Xian, L.; Lei, Q.; Song, J.; Zhong, J. Nrf2 Overexpression for the Protective Effect of Skin-Derived Precursors against Uv-Induced Damage: Evidence from a Three-Dimensional Skin Model. Oxidative Med. Cell. Longev. 2019, 2019, 7021428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, H.; Dumont, M.J.; Simpson, B.K. Extraction of Gelatin from Salmon (Salmo Salar) Fish Skin Using Trypsin-Aided Process: Optimization by Plackett–Burman and Response Surface Methodological Approaches. J. Food Sci. Technol. 2017, 54, 4000–4008. [Google Scholar] [CrossRef]









| Gene | Sequence |
|---|---|
| Gapdh | F: 5′- TCGACAGTCAGCCGCATCTTCTTT -3′ R: 5′- ACCAAATCCGTTGACTCCGACCTT -3′ |
| IL-6 | F: 5′- CACAGACAGCCACTCACCTC -3′ R: 5′- TTTTCTGCCAGTGCCTCTTT -3′ |
| IL1-β | F: 5′- GGACAAGCTGAGGAAGATGC -3′ R: 5′- TCGTTATCCCATGTGTGTCGAA -3′ |
| TNF-α | F: 5′- CAAAGTAGACCTGCCCAGAC -3′ R: 5′- GACCTCTCTCTAATCAGCCC -3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S.H.; Ballinger, E.; Choung, S.-Y.; Kwon, J.Y. Anti-Photoaging Effect of Hydrolysates from Pacific Whiting Skin via MAPK/AP-1, NF-κB, TGF-β/Smad, and Nrf-2/HO-1 Signaling Pathway in UVB-Induced Human Dermal Fibroblasts. Mar. Drugs 2022, 20, 308. https://doi.org/10.3390/md20050308
Han SH, Ballinger E, Choung S-Y, Kwon JY. Anti-Photoaging Effect of Hydrolysates from Pacific Whiting Skin via MAPK/AP-1, NF-κB, TGF-β/Smad, and Nrf-2/HO-1 Signaling Pathway in UVB-Induced Human Dermal Fibroblasts. Marine Drugs. 2022; 20(5):308. https://doi.org/10.3390/md20050308
Chicago/Turabian StyleHan, Seok Hee, Elaine Ballinger, Se-Young Choung, and Jung Yeon Kwon. 2022. "Anti-Photoaging Effect of Hydrolysates from Pacific Whiting Skin via MAPK/AP-1, NF-κB, TGF-β/Smad, and Nrf-2/HO-1 Signaling Pathway in UVB-Induced Human Dermal Fibroblasts" Marine Drugs 20, no. 5: 308. https://doi.org/10.3390/md20050308