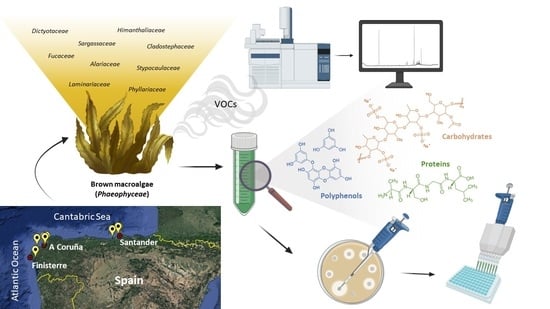
Brown Macroalgae (Phaeophyceae): A Valuable Reservoir of Antimicrobial Compounds on Northern Coast of Spain
Abstract
:1. Introduction
2. Results and Discussion
2.1. Volatile Compounds in the Biomass of Seaweeds
2.2. Chemical Composition of Extracts
2.3. Antimicrobial Activity of Macroalgae Extracts
3. Materials and Methods
3.1. Macroalgae Samples Collection and Processing
3.2. Headspace Solid-Phase Microextraction (HS-SPME) and GC-MS Conditions
3.3. Extraction Procedure
3.4. Determination of Proximate Composition
3.4.1. Total Phenolic Content
3.4.2. Carbohydrate Content
3.4.3. Protein Content
3.5. Antimicrobial Activity Screening
3.5.1. Test Microorganisms and Culture Conditions
3.5.2. Disk Diffusion Assay
3.5.3. Determination of MIC (Minimum Inhibitory Concentration) and MBC (Minimum Bactericidal Concentration)
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tait, L.W.; Schiel, D.R. Ecophysiology of layered macroalgal assemblages: Importance of subcanopy species biodiversity in buffering primary production. Front. Mar. Sci. 2018, 5, 444. [Google Scholar] [CrossRef]
- Lalegerie, F.; Gager, L.; Stiger-Pouvreau, V.; Connan, S. Chapter eight—The stressful life of red and brown seaweeds on the temperate intertidal zone: Effect of abiotic and biotic parameters on the physiology of macroalgae and content variability of particular metabolites. In Seaweeds Around the World: State of Art and Perspectives; Bourgougnon, N., Ed.; Academic Press: Cambidge, MA, USA, 2020; Volume 95, pp. 247–287. [Google Scholar] [CrossRef]
- Andrade, P.B.; Barbosa, M.; Matos, R.P.; Lopes, G.; Vinholes, J.; Mouga, T.; Valentão, P. Valuable compounds in macroalgae extracts. Food Chem. 2013, 138, 1819–1828. [Google Scholar] [CrossRef]
- Martínez, M.A.; Ares, I.; Martínez, M.; Lopez-Torres, B.; Maximiliano, J.E.; Rodríguez, J.L.; Martínez-Larrañaga, M.R.; Anadón, A.; Peteiro, C.; Rubiño, S.; et al. Brown marine algae Gongolaria baccata extract protects Caco-2 cells from oxidative stress induced by tert-butyl hydroperoxide. Food Chem. Toxicol. 2021, 156, 112460. [Google Scholar] [CrossRef] [PubMed]
- Michalak, I.; Chojnacka, K. Algae as production systems of bioactive compounds. Eng. Life Sci. 2015, 15, 160–176. [Google Scholar] [CrossRef]
- Bondi, M.; Lauková, A.; De Niederhausern, S.; Messi, P.; Papadopoulou, C. Natural preservatives to improve food quality and safety. J. Food Qual. 2017, 2017, 1090932. [Google Scholar] [CrossRef] [Green Version]
- Kostas, E.T.; Adams, J.M.M.; Ruiz, H.A.; Durán-Jiménez, G.; Lye, G.J. Macroalgal biorefinery concepts for the circular bioeconomy: A review on biotechnological developments and future perspectives. Renew. Sustain. Energy Rev. 2021, 151, 111553. [Google Scholar] [CrossRef]
- Pereira, R.; Yarish, C. Mass production of marine macroalgae. In Encyclopedia of Ecology; Five-Volume Set; Academic Press: Cambridge, MA, USA, 2008; pp. 2236–2247. [Google Scholar] [CrossRef]
- Chung, I.K.; Beardall, J.; Mehta, S.; Sahoo, D.; Stojkovic, S. Using marine macroalgae for carbon sequestration: A critical appraisal. J. Appl. Phycol. 2011, 23, 877–886. [Google Scholar] [CrossRef]
- Peteiro, C. Alginate production from marine macroalgae, with emphasis on kelp farming. In Alginates and Their Biomedical Applications; Rehm, B.H.A., Moradali, M.F., Eds.; Springer: Singapore, 2018; Volume 11, pp. 27–66. [Google Scholar] [CrossRef]
- Zollmann, M.; Rubinsky, B.; Liberzon, A.; Golberg, A. Multi-Scale modeling of intensive macroalgae cultivation and marine nitrogen sequestration. Commun. Biol. 2021, 4, 848. [Google Scholar] [CrossRef]
- European Parliament; Council of the European Union. Commission Implementing Regulation (EU) 2017/2470 of 20 December 2017 establishing the Union list of novel foods in accordance with Regulation (EU) 2015/2283 of the European Parliament and of the Council on novel foods. Off. J. Eur. Union 2017, 351, 72–201. [Google Scholar]
- Araújo, R.; Peteiro, C. Algae as Food and Food Supplements in Europe, Luxembourg, Technical Report by the Joint Research Centre (JRC), Publications Office of the European Union. 2021. Available online: https://data.europa.eu/doi/10.2760/049515 (accessed on 8 October 2022).
- Bassetti, M.; Giacobbe, D.R. A Look at the clinical, economic, and societal impact of antimicrobial resistance in 2020. Expert Opin. Pharmacother. 2020, 21, 2067–2071. [Google Scholar] [CrossRef]
- Subramaniam, G.; Girish, M. Antibiotic resistance—A cause for reemergence of infections. Indian J. Pediatr. 2020, 87, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Silva, S.A.; Carpena, M.; Garcia-Oliveira, P.; Gullón, P.; Barroso, M.F.; Prieto, M.A.; Simal-Gandara, J. Macroalgae as a source of valuable antimicrobial compounds: Extraction and applications. Antibiotics 2020, 9, 642. [Google Scholar] [CrossRef] [PubMed]
- Hafting, J.T.; Craigie, J.S.; Stengel, D.B.; Loureiro, R.R.; Buschmann, A.H.; Yarish, C.; Edwards, M.D.; Critchley, A.T. Prospects and challenges for industrial production of seaweed bioactives. J. Phycol. 2015, 51, 821–837. [Google Scholar] [CrossRef] [PubMed]
- Ramos, E.; Puente, A.; Juanes, J.A. An ecological classification of rocky shores at a regional scale: A predictive tool for management of conservation values. Mar. Ecol. 2016, 37, 311–328. [Google Scholar] [CrossRef]
- Barbier, M.; Charrier, B.; Araujo, R.; Holdt, S.L.; Jacquemin, B.; Rebours, C. Pegasus—Phycomorph European guidelines for a sustainable aquaculture of seaweeds. In COST Action FA1406; Barbier, M., Charrier, B., Eds.; European Cooperation in Science and Technology: Roscoff, France, 2019. [Google Scholar] [CrossRef]
- Garicano Vilar, E.; O’Sullivan, M.G.; Kerry, J.P.; Kilcawley, K.N. Volatile compounds of six species of edible seaweed: A review. Algal Res. 2020, 45, 101740. [Google Scholar] [CrossRef]
- Boonprab, K.; Matsui, K.; Akakabe, Y.; Yoshida, M.; Yotsukura, N.; Chirapart, A.; Kajiwara, T. Formation of aldehyde flavor (n-hexanal, 3Z-nonenal and 2E-nonenal) in the brown alga, Laminaria angustata. J. Appl. Phycol. 2006, 18, 409–412. [Google Scholar] [CrossRef]
- Matsui, K. Green leaf volatiles: Hydroperoxide lyase pathway of oxylipin metabolism. Curr. Opin. Plant Biol. 2006, 9, 274–280. [Google Scholar] [CrossRef]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of plant-derived flavor compounds. Plant J. 2008, 54, 712–732. [Google Scholar] [CrossRef]
- Scala, A.; Allmann, S.; Mirabella, R.; Haring, M.A.; Schuurink, R.C. Green leaf volatiles: A plant’s multifunctional weapon against herbivores and pathogens. Int. J. Mol. Sci. 2013, 14, 17781–17811. [Google Scholar] [CrossRef] [Green Version]
- Lanciotti, R.; Belletti, N.; Patrignani, F.; Gianotti, A.; Gardini, F.; Guerzoni, M.E. Application of hexanal, (E)-2-hexenal, and hexyl acetate to improve the safety of fresh-sliced apples. J. Agric. Food Chem. 2003, 51, 2958–2963. [Google Scholar] [CrossRef]
- Laturnus, F.; Giese, B.; Wiencke, C.; Adams, F.C. Low-molecular-weight organoiodine and organobromine compounds released by polar macroalgae—The influence of abiotic factors. Fresenius J. Anal. Chem. 2000, 368, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Ohsawa, N.; Ogata, Y.; Okada, N.; Itoh, N. Physiological function of bromoperoxidase in the red marine alga, Corallina pilulifera: Production of bromoform as an allelochemical and the simultaneous elimination of hydrogen peroxide. Phytochemistry 2001, 58, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Glasson, C.R.; Kinley, R.D.; de Nys, R.; King, N.; Adams, S.L.; Packer, M.A.; Svenson, J.; Eason, C.T.; Magnusson, M. Benefits and risks of including the bromoform containing seaweed Asparagopsis in feed for the reduction of methane production from ruminants. Algal Res. 2022, 64, 102673. [Google Scholar] [CrossRef]
- Romanazzi, D.; Sanchez-Garcia, C.; Svenson, J.; Mata, L.; Pes, K.; Hayman, C.M.; Wheeler, T.T.; Magnusson, M. Rapid analytical method for the quantification of bromoform in the red seaweeds Asparagopsis armata and Asparagopsis taxiformis using gas chromatography–mass spectrometry. ACS Agric. Sci. Technol. 2021, 1, 436–442. [Google Scholar] [CrossRef]
- Bravo-Linares, C.M.; Mudge, S.M.; Loyola-Sepulveda, R.H. Production of volatile organic compounds (VOCs) by temperate macroalgae. The use of Solid Phase Microextraction (SPME) coupled to GC-MS as method of analysis. J. Chil. Chem. Soc. 2010, 55, 227–232. [Google Scholar] [CrossRef] [Green Version]
- Hakim, M.M.; Patel, I.C. A review on phytoconstituents of marine brown algae. Future J. Pharm. Sci. 2020, 6, 129. [Google Scholar] [CrossRef]
- Eom, S.H.; Kim, Y.M.; Kim, S.K. Antimicrobial effect of phlorotannins from marine brown algae. Food Chem. Toxicol. 2012, 50, 3251–3255. [Google Scholar] [CrossRef]
- Karpiński, T.M.; Adamczak, A. Fucoxanthin—An antibacterial carotenoid. Antioxidants 2019, 8, 239. [Google Scholar] [CrossRef] [Green Version]
- Ayrapetyan, O.N.; Obluchinskaya, E.D.; Zhurishkina, E.V.; Skorik, Y.A.; Lebedev, D.V.; Kulminskaya, A.A.; Lapina, I.M. Antibacterial properties of fucoidans from the brown algae Fucus vesiculosus L. of the Barents Sea. Biology 2021, 10, 67. [Google Scholar] [CrossRef]
- Sosa-Hernández, J.E.; Escobedo-Avellaneda, Z.; Iqbal, H.M.N.; Welti-Chanes, J. State-of-the-art extraction methodologies for bioactive compounds from algal biome to meet bio-economy challenges and opportunities. Molecules 2018, 23, 2953. [Google Scholar] [CrossRef] [Green Version]
- Lann, K.L.; Ferret, C.; VanMee, E.; Spagnol, C.; Lhuillery, M.; Payri, C.; Stiger-Pouvreau, V. Total phenolic, size-fractionated phenolics and fucoxanthin content of tropical Sargassaceae (Fucales, Phaeophyceae) from the South Pacific Ocean: Spatial and specific variability. Phycol. Res. 2012, 60, 37–50. [Google Scholar] [CrossRef]
- Catarino, M.D.; Silva, A.M.S.; Cardoso, S.M. Fucaceae: A source of bioactive phlorotannins. Int. J. Mol. Sci. 2017, 18, 1327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stiger-Pouvreau, V.; Jégou, C.; Cérantola, S.; Guérard, F.; Le Lann, K. Chapter Thirteen—Phlorotannins in Sargassaceae species from Brittany (France): Interesting molecules for ecophysiological and valorisation purposes. In Sea Plants; Bourgougnon, N., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 71, pp. 379–411. [Google Scholar] [CrossRef]
- Jacobsen, J.; Sørensen, A.D.M.; Holdt, S.L.; Akoh, C.C.; Hermund, D.B. Source, extraction, characterization, and applications of novel antioxidants from seaweed. Annu. Rev. Food Sci. Technol. 2019, 10, 541–568. [Google Scholar] [CrossRef] [PubMed]
- Tierney, M.S.; Smyth, T.J.; Rai, D.K.; Soler-Vila, A.; Croft, A.K.; Brunton, N. Enrichment of polyphenol contents and antioxidant activities of Irish brown macroalgae using food-friendly techniques based on polarity and molecular size. Food Chem. 2013, 139, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Kadam, S.U.; O’Donnell, C.P.; Rai, D.K.; Hossain, M.B.; Burgess, C.M.; Walsh, D.; Tiwari, B.K. Laminarin from Irish brown seaweeds Ascophyllum nodosum and Laminaria hyperborea: Ultrasound Assisted Extraction, characterization and bioactivity. Mar. Drugs 2015, 13, 4270–4280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Generalić Mekinić, I.; Skroza, D.; Šimat, V.; Hamed, I.; Čagalj, M.; Popović Perković, Z. Phenolic content of brown algae (Phaeophyceae) species: Extraction, identification, and quantification. Biomolecules 2019, 9, 244. [Google Scholar] [CrossRef]
- Rajauria, G.; Jaiswal, A.K.; Abu-Ghannam, N.; Gupta, S. Effect of hydrothermal processing on colour, antioxidant and free radical scavenging capacities of edible Irish brown seaweeds. Int. J. Food Sci. Technol. 2010, 45, 2485–2493. [Google Scholar] [CrossRef]
- Belda, M.; Sanchez, D.; Bover, E.; Prieto, B.; Padrón, C.; Cejalvo, D.; Lloris, J.M. Extraction of polyphenols in Himanthalia elongata and determination by High Performance Liquid Chromatography with Diode Array Detector prior to its potential use against oxidative stress. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1033–1034, 334–341. [Google Scholar] [CrossRef]
- Heffernan, N.; Smyth, T.J.; Fitzgerald, R.J.; Soler-Vila, A.; Brunton, N. Antioxidant activity and phenolic content of pressurised liquid and solid—liquid extracts from four Irish origin macroalgae. Int. J. Food Sci. Technol. 2014, 49, 1765–1772. [Google Scholar] [CrossRef]
- Olsson, J.; Toth, G.B.; Albers, E. Biochemical composition of red, green and brown seaweeds on the Swedish West coast. J. Appl. Phycol. 2020, 32, 3305–3317. [Google Scholar] [CrossRef]
- Saifullah; Olsen, Y.; Surilayani, D.; Handå, A. Carbohydrate of the brown seaweed, Saccharina latissima: A review. In Joint Proceedings of the 2nd and the 3rd International Conference on Food Security Innovation; Atlantis Press: Amsterdam, The Netherlands, 2021; pp. 31–34. [Google Scholar] [CrossRef]
- Garcia-Oliveira, P.; Carreira-Casais, A.; Caleja, C.; Pereira, E.; Calhelha, R.C.; Sokovic, M.; Simal-Gandara, J.; Ferreira, I.C.F.R.; Prieto, M.A.; Barros, L. Macroalgae as an alternative source of nutrients and compounds with bioactive potential. Proceedings 2020, 70, 46. [Google Scholar] [CrossRef]
- Otero, P.; Carpena, M.; Garcia-Oliveira, P.; Echave, J.; Soria-Lopez, A.; Garcia-Perez, P.; Fraga-Corral, M.; Cao, H.; Nie, S.; Xiao, J.; et al. Seaweed polysaccharides: Emerging extraction technologies, chemical modifications and bioactive properties. Crit. Rev. Food Sci. Nutr. 2021, 61, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vaquero, M.; O’Doherty, J.V.; Tiwari, B.K.; Sweeney, T.; Rajauria, G. Enhancing the extraction of polysaccharides and antioxidants from macroalgae using sequential Hydrothermal-Assisted Extraction followed by ultrasound and thermal technologies. Mar. Drugs. 2019, 17, 457. [Google Scholar] [CrossRef] [Green Version]
- Torres, M.D.; Kraan, S.; Domínguez, H. Seaweed Biorefinery. Rev. Environ. Sci. Biotechnol. 2019, 18, 335–388. [Google Scholar] [CrossRef]
- Lemesheva, V.; Tarakhovskaya, E. Physiological functions of phlorotannins. Biol. Commun. 2018, 63, 70–76. [Google Scholar] [CrossRef]
- Schiener, P.; Black, K.D.; Stanley, M.S.; Green, D.H. The seasonal variation in the chemical composition of the kelp species Laminaria digitata, Laminaria hyperborea, Saccharina latissima and Alaria esculenta. J. Appl. Phycol. 2015, 27, 363–373. [Google Scholar] [CrossRef]
- Garcia-Vaquero, M.; Rajauria, G.; Miranda, M.; Sweeney, T.; Lopez-Alonso, M.; O’Doherty, J. Seasonal variation of the proximate composition, mineral content, fatty acid profiles and other phytochemical constituents of selected brown macroalgae. Mar. Drugs 2021, 19, 204. [Google Scholar] [CrossRef]
- Dodd, C.E.; Aldsworth, T.G.; Stein, R.A. (Eds.) Foodborne Diseases; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Kakagianni, M.; Koutsoumanis, K.P. Mapping the risk of evaporated milk spoilage in the Mediterranean region based on the effect of temperature conditions on Geobacillus stearothermophilus growth. Food Res. Int. 2018, 111, 104–110. [Google Scholar] [CrossRef]
- Miller, S.I. Antibiotic resistance and regulation of the Gram-negative bacterial outer membrane barrier by host innate immune molecules. MBio. 2016, 7, e0154116. [Google Scholar] [CrossRef] [Green Version]
- El Wahidi, M.; El Amraoui, B.; El Amraoui, M.; Bamhaoud, T. Screening of antimicrobial activity of macroalgae extracts from the Moroccan Atlantic coast. Ann. Pharm. Françaises 2015, 73, 190–196. [Google Scholar] [CrossRef]
- Alves, C.; Pinteus, S.; Simões, T.; Horta, A.; Silva, J.; Tecelão, C.; Pedrosa, R. Bifurcaria bifurcata: A key macro-alga as a source of bioactive compounds and functional ingredients. Int. J. Food Sci. Technol. 2016, 51, 1638–1646. [Google Scholar] [CrossRef]
- Fernández-No, I.C.; Guarddon, M.; Böhme, K.; Cepeda, A.; Calo-Mata, P.; Barros-Velázquez, J. Detection and quantification of spoilage and pathogenic Bacillus cereus, Bacillus subtilis and Bacillus licheniformis by real-time PCR. Food Microbiol. 2011, 28, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Bremer, P.; Seale, B.; Flint, S.; Palmer, J. Biofilms in dairy processing. In Biofilms in the Food and Beverage Industries; Woodhead Publishing Series in Food Science, Technology and Nutrition; Fratamico, P.M., Annous, B.A., Gunther, N.W., Eds.; Woodhead Publishing: Sawston, UK, 2009; pp. 396–431. [Google Scholar] [CrossRef]
- Burgess, S.A.; Flint, S.H.; Lindsay, D.; Cox, M.P.; Biggs, P.J. Insights into the Geobacillus stearothermophilus species based on phylogenomic principles. BMC Microbiol. 2017, 17, 140. [Google Scholar] [CrossRef] [PubMed]
- Allende, A.; Bolton, D.; Chemaly, M.; Davies, R.; Fernández Escámez, P.S.; Gironés, R.; Herman, L.; Koutsoumanis, K.; Lindqvist, R.; Nørrung, B.; et al. Risks for public health related to the presence of Bacillus cereus and other Bacillus spp. including Bacillus thuringiensis in foodstuffs. EFSA J. 2016, 14, e4524. [Google Scholar] [CrossRef]
- Griffiths, M.W.; Schraft, H. Chapter 20—Bacillus cereus Food Poisoning. In Foodborne Diseases, 3rd ed.; Dodd, C.E.R., Aldsworth, T., Stein, R.A., Cliver, D.O., Riemann, H.P., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 395–405. [Google Scholar] [CrossRef]
- Kimura, K.; Yokoyama, S. Trends in the application of Bacillus in fermented foods. Curr. Opin. Biotechnol. 2019, 56, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Pavic, S.; Brett, M.; Petric, N.; Lastre, D.; Smoljanovic, M.; Atkinson, M. An outbreak of food poisoning in a kindergarten caused by milk powder containing toxigenic Bacillus subtilis and Bacillus licheniformis. Arch. Lebensmittelhygiene 2005, 56, 20–22. [Google Scholar]
- Burgess, S.A.; Lindsay, D.; Flint, S.H. Thermophilic bacilli and their importance in dairy processing. Int. J. Food Microbiol. 2010, 144, 215–225. [Google Scholar] [CrossRef]
- Fayzi, L.; Askarne, L.; Cherifi, O.; Boufous, E.H.; Cherifi, K. Comparative antibacterial activity of some selected seaweed extracts from Agadir coastal regions in Morocco. Int. J. Curr. Microbiol. App. Sci. 2020, 9, 390–399. [Google Scholar] [CrossRef]
- Salvador, N.; Garreta, A.G.; Lavelli, L.; Ribera, M.A. Antimicrobial activity of Iberian macroalgae. Sci. Mar. 2007, 71, 101–114. [Google Scholar] [CrossRef] [Green Version]
- Götz, F.; Bannerman, T.; Schleifer, K.H. The genera Staphylococcus and Macrococcus. Prokaryotes 2006, 4, 5–75. [Google Scholar] [CrossRef]
- Tong, S.Y.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G., Jr. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Argudín, M.A.; Mendoza, M.C.; Rodicio, M.R. Food poisoning and Staphylococcus aureus enterotoxins. Toxins 2010, 2, 1751–1773. [Google Scholar] [CrossRef] [PubMed]
- Karima, S.; Fatiha, B. Study of the antimicrobial activity of four algerian marin algae species. In Microbes in Applied Research; World Scientific: Singapore, 2012; pp. 578–581. [Google Scholar]
- Johnsi-Christobel, G.; Lipton, A.P.; Aishwarya, M.S.; Sarika, A.R.; Udayakumar, A. Antibacterial activity of aqueous extract from selected macroalgae of Southwest coast of India. Seaweed Res. Util. 2011, 33, 67–75. [Google Scholar] [CrossRef]
- Zouaoui, B.; Ghalem, B.R. The phenolic contents and antimicrobial activities of some marine algae from the Mediterranean Sea (Algeria). Russ. J. Mar. Biol. 2017, 43, 491–495. [Google Scholar] [CrossRef]
- Kosanić, M.; Ranković, B.; Stanojković, T. Brown macroalgae from the Adriatic Sea as a promising source of bioactive nutrients. J. Food Meas. Charact. 2019, 13, 330–338. [Google Scholar] [CrossRef]
- Eloff, J.N. Avoiding pitfalls in determining antimicrobial activity of plant extracts and publishing the results. BMC Complement. Altern. Med. 2019, 19, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Cabral, E.M.; Mondala, J.M.R.; Oliveira, M.; Przyborska, J.; Fitzpatrick, S.; Rai, D.K.; Sivagnanam, S.P.; Garcia-Vaquero, M.; O’Shea, D.; Devereux, M.; et al. Influence of molecular weight fractionation on the antimicrobial and anticancer properties of a fucoidan rich-extract from the macroalgae Fucus vesiculosus. Int. J. Biol. Macromol. 2021, 186, 994–1002. [Google Scholar] [CrossRef]
- Rubiño, S.; Peteiro, C.; Aymerich, T.; Hortós, M. Major lipophilic pigments in Atlantic seaweeds as valuable food ingredients: Analysis and assessment of quantification methods. Food Res. Int. 2022, 159, 111609. [Google Scholar] [CrossRef]
- Čagalj, M.; Skroza, D.; Razola-Díaz, M.d.C.; Verardo, V.; Bassi, D.; Frleta, R.; Generalić Mekinić, I.; Tabanelli, G.; Šimat, V. Variations in the composition, antioxidant and antimicrobial activities of Cystoseira compressa during seasonal growth. Mar. Drugs. 2022, 20, 64. [Google Scholar] [CrossRef]
- Karkhaneh Yousefi, M.; Seyed Hashtroudi, M.; Mashinchian Moradi, A.; Ghassempour, A.R. Seasonal variation of fucoxanthin content in four species of brown seaweeds from Qeshm Island, Persian Gulf and evaluation of their antibacterial and antioxidant activities. Iran. J. Fish. Sci. 2020, 19, 2394–2408. [Google Scholar] [CrossRef]
- Turland, N.J.; Wiersema, J.H.; Barrie, F.R.; Greuter, W.; Hawksworth, D.L.; Herendeen, P.S.; Knapp, S.; Kusber, W.H.; Li, D.Z.; Marhold, K.; et al. (Eds.) International Code of Nomenclature for Algae, Fungi, and Plants (Shenzhen Code) Adopted by the Nineteenth) International Botanical Congress Shenzhen, China, July 2017; Regnum Vegetabile; Koeltz Botanical Books: Glashütten, Germany, 2018; Volume 159. [Google Scholar] [CrossRef]
- European Parliament; Council of the European Union. Directive 2009/32/EC of the European Parliament and of the Council of 23 April 2009 on the approximation of the laws of the Member States on extraction solvents used in the production of foodstuffs and food ingredients. Off. J. Eur. Union 2009, 141, 3–11. [Google Scholar]
- Zhang, Q.; Zhang, J.; Shen, J.; Silva, A.; Dennis, D.A.; Barrow, C.J. A Simple 96-well microplate method for estimation of total polyphenol content in seaweeds. J. Appl. Phycol. 2006, 18, 445–450. [Google Scholar] [CrossRef] [Green Version]
- Long, G.L.; Winefordner, J. Limit of detection. A closer look at the IUPAC definition. In Statistics for Environmental Engineers, 2nd ed.; Lewis Publishers: Boca Raton, FL, USA, 1983; Volume 55, pp. 712A–724A. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.; Hamilton, J.K.; Rebers, P.A.; Smith, F. A colorimetric method for the determination of sugars. Nature 1951, 168, 167. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Test for Bacteria that Grow Aerobically, 11th ed.; Approved Standard, CLSI Standard M07; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]


| Compound | Rt | Compound Class | Formula | Ala | Cla | Dic | Fuc | Him | Lam | Phy | Sar | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Up | Cs | Dd | Pc | An | Fg | Fc | Fs | Fv | Fsp | He | Sl | Lh | Sp | Bb | Gb | Sm | Es | ||||
| 1-Penten-3-ol | 7.73 | Alcohol | C5H10O | √ | √ | √ | √ | √ | |||||||||||||
| 1-Penten-3-one | 7.82 | Ketone | C5H8O | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | ||||||
| 2-Penten-1-ol | 14.06 | Alcohol | C5H10O | √ | √ | √ | √ | √ | √ | √ | √ | ||||||||||
| 3-Penten-2-one-4-methyl | 15.89 | Ketone | C6H10O | √ | |||||||||||||||||
| Hexanal | 16.17 | Aldehyde | C6H12O | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | ||||||||
| 2-Hexenal | 19.43 | Aldehyde | C6H10O | √ | √ | √ | √ | √ | |||||||||||||
| 1-Hexen-3-ol | 19.52 | Alcohol | C6H12O | √ | √ | √ | √ | √ | √ | √ | |||||||||||
| 1-Hexanol | 20.30 | Alcohol | C6H14O | √ | √ | √ | √ | √ | √ | √ | √ | √ | |||||||||
| Methane, tribrome | 21.71 | Halogenated compound | CHBr3 | √ | √ | √ | |||||||||||||||
| Butane-1-iodo-3-methyl | 23.41 | Halogenated compound | C5H11I | √ | √ | ||||||||||||||||
| 2-Hexene-3,5,5-trimethyl | 25.80 | Hydrocarbon | C9H18 | √ | √ | √ | |||||||||||||||
| 1-Octen-3-one | 25.96 | Ketone | C8H14O | √ | √ | √ | √ | √ | √ | √ | |||||||||||
| 1-Octen-3-ol | 26.08 | Alcohol | C8H16O | √ | √ | √ | √ | √ | √ | ||||||||||||
| 4-Hexen-1-ol, acetate | 27.21 | Ester | C8H14O | √ | |||||||||||||||||
| 2-Octenal | 29.71 | Aldehyde | C8H14O | √ | |||||||||||||||||
| 2-Octen-1-ol | 30.01 | Alcohol | C8H16O | √ | √ | ||||||||||||||||
| 2,7-Octadien-1-ol | 30.12 | Alcohol | C8H14O | √ | |||||||||||||||||
| 2,6-Nonadienal | 33.68 | Aldehyde | C9H14O | √ | |||||||||||||||||
| 2-Nonenal | 33.95 | Aldehyde | C9H16O | √ | |||||||||||||||||
| Benzene-1,3-dimethoxy | 34.31 | Aromatic compound | C8H10O | √ | |||||||||||||||||
| Pentadecane | 50.50 | Hydrocarbon | C15H32 | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | ||||||
| Tridecanal | 50.79 | Aldehyde | C13H26O | √ | √ | √ | |||||||||||||||
| o-Hydroxybiphenil | 51.12 | Aromatic compound | C12H10O | √ | |||||||||||||||||
| Standard | Wavelength | Equations | R2 | Linear Range | LOD | LOQ |
|---|---|---|---|---|---|---|
| Phloroglucinol | 765 nm | Abs = 17.768 * μg + 0.203 | 0.999 | 1–8 μg | 0.14 μg | 0.47 μg |
| Glucose | 490 nm | Abs = 43.955 * μg − 2.508 | 0.997 | 5–50 μg | 2.66 μg | 8.86 μg |
| Bovine serum albumin | 595 nm | Abs = 45.398 * μg − 1.430 | 0.998 | 5–40 μg | 1.59 μg | 5.30 μg |
| Order, Family/Species | Month/Year | Phenolics 1 | Carbohydrate 1 | Protein 1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mg/g dw | SD | mg/g dw | SD | mg/g dw | SD | ||||||||
| Dictyotales, Dictyotaceae | |||||||||||||
| Dictyota dichotoma | September 2019 | 19.73 | f,g,h | ± | 1.93 | 11.10 | d,e,f | ± | 0.75 | ≤2.78 | |||
| Fucales, Fucaceae | |||||||||||||
| Ascophyllum nodosum | August 2019 | 42.64 | b,c | ± | 9.04 | 15.14 | b,c | ± | 1.06 | 5.33 | b,c,d,e | ± | 0.63 |
| Fucus ceranoides | November 2019 | 8.44 | g,h,i | ± | 0.62 | 19.15 | c | ± | 0.66 | ≤2.78 | |||
| Fucus guiryi | August 2017 | 2.45 | i | ± | 0.06 | ≤4.65 | 6.79 | a,b,c,d,e | ± | 0.55 | |||
| September 2019 | 29.33 | d,e,f | ± | 3.58 | 7.71 | e,f,g,h | ± | 0.63 | 7.14 | a,b,c,d,e | ± | 0.89 | |
| Fucus serratus | November 2019 | 43.87 | b,c | ± | 5.12 | 15.61 | c,d | ± | 2.65 | 8.52 | a,b,c | ± | 0.58 |
| Fucus spiralis | November 2019 | 20.10 | f,g,h | ± | 1.61 | 10.68 | d,e,f,g,h | ± | 0.07 | 5.70 | b,c,d,e, | ± | 0.76 |
| December 2019 | 24.89 | d,e,f,g | ± | 1.19 | ≤4.65 | 5.85 | b,c,d,e | ± | 0.5 | ||||
| Fucus vesiculosus | November 2019 | 54.58 | b | ± | 1.25 | 13.57 | b,c | ± | 0.86 | 5.93 | b,c,d,e | ± | 0.52 |
| Pelvetia canaliculata | August 2017 | 3.17 | i | ± | 0.26 | 6.12 | g,h | ± | 0.65 | 10.03 | a,b | ± | 1.48 |
| August 2019 | 14.06 | g,h,i | ± | 0.70 | 5.15 | h | ± | 0.25 | 6.36 | a,b,c,d,e | ± | 0.69 | |
| Fucales, Himanthaliaceae | |||||||||||||
| Himanthalia elongata | May 2019 | 48.31 | b, | ± | 2.49 | 6.69 | f,g,h | ± | 1.03 | 10.74 | a | ± | 0.40 |
| August 2019 | 25.47 | d,e,f,g | ± | 1.78 | 5.51 | g,h | ± | 0.23 | 8.40 | a,b,c,d | ± | 0.37 | |
| Fucales, Sargassaceae | |||||||||||||
| Bifurcaria bifurcata | August 2017 | 20.87 | e,f,g,h | ± | 1.89 | 12.91 | d,e | ± | 1.70 | ≤2.78 | |||
| May 2019 (vegetative) | 24.01 | d,e,f,g | ± | 2.41 | 9.62 | e,f,g,h | ± | 1.42 | ≤2.78 | ||||
| May 2019 (fertile) | 33.40 | c,d,e | ± | 2.24 | 10.43 | d,e,f,g,h | ± | 1.31 | 3.67 | d,e | ± | 1.10 | |
| December 2019 | 19.28 | f,g,h | ± | 1.60 | 11.34 | d,e,f,g | ± | 2.51 | ≤2.78 | ||||
| Ericaria selaginoides | August 2017 | 23.06 | d,e,f,g | ± | 1.33 | 12.16 | d,e,f | ± | 1.61 | ≤2.78 | |||
| August 2019 | 24.74 | d,e,f,g | ± | 3.37 | 9.21 | e,f,g,h | ± | 0.68 | ≤2.78 | ||||
| Gongolaria baccata | August 2017 | 34.63 | c,d, | ± | 2.60 | 8.74 | e,f,g,h | ± | 1.33 | 3.57 | e | ± | 0.27 |
| Halidrys siliquosa | November 2019 | 115.13 | a | ± | 16.36 | 18.95 | b | ± | 2.04 | 6.60 | a,b,c,d,e | ± | 1.59 |
| Sargassum muticum | May 2019 | 13.31 | g,h,i | ± | 0.51 | 6.05 | g,h | ± | 0.59 | ≤2.78 | |||
| Laminariales, Alariaceae | |||||||||||||
| Undaria pinnatifida | May 2019 | 6.25 | i | ± | 0.19 | 7.76 | e,f,g,h | ± | 0.55 | 3.76 | c,d,e | ± | 5.39 |
| Laminariales, Laminariaceae | |||||||||||||
| Saccharina latissima | August 2019 | 2.52 | i | ± | 0.06 | 33.43 | a | ± | 2.02 | nd, | |||
| Laminaria hyperborea | August 2019 | 2.28 | i | ± | 0.12 | 11.30 | d,e,f,g | ± | 0.33 | ≤2.78 | |||
| Sphacelariales, Cladostephaceae | |||||||||||||
| Cladostephus spongiosus | September 2019 | 5.25 | i | ± | 0.40 | 9.93 | d,e,f,g,h | ± | 0.65 | nd | |||
| Tilopteridales, Phyllariaceae | |||||||||||||
| Sacchoriza polyschides | May 2019 | 4.60 | i | ± | 1.10 | ≤4.65 | nd | ||||||
| Macroalgae Species | Month/Year Collection | Location Collection | Test Organisms—Inhibition Zone (mm) | |||||
|---|---|---|---|---|---|---|---|---|
| Bc | Bs | Gs | Lm | Sa | Sh | |||
| Bifurcaria bifurcata | August 2017 | Cantabria | 13.6 ± 0.5 | 13.0 ± 0.0 | 17.6 ± 0.0 | 18.3 ± 1.0 | 13.6 ± 0.4 | 15.3 ± 2.3 |
| May 2019 | Galicia | nd | nd | 15.7 ± 0.0 | 18.4 ± 0.6 | 13.5 ± 0.4 | +/− | |
| May 2019 | Galicia | nd | nd | 14.6 ± 0.0 | 17.1 ± 1.5 | +/− | nd | |
| December 2019 | Cantabria | 12.7 ± 0.5 | 11.2 ± 0.8 | 18.3 ± 0.0 | 19.2 ± 0.4 | +/− | 14.5 ± 0.2 | |
| Ericaria selaginoides | August 2017 | Cantabria | 11.7 ± 0.6 | 11.5 ± 0.9 | 13.4 ± 0.0 | 13.9 ± 1.4 | 12.8 ± 0.0 | nd |
| August 2019 | Galicia | nd | nd | +/− | nd | +/− | +/− | |
| Dictyota dichotoma | September 2019 | Cantabria | 10.4 ± 0.0 | 12.4 ± 0.2 | 15.2 ± 0.0 | 10.4 ± 0.0 | +/− | nd |
| Macroalgae Species | Month/Year Collection | Location Collection | Test Organisms—MIC/MBC (mg/mL) | |||||
|---|---|---|---|---|---|---|---|---|
| Bc | Bs | Gs | Lm | Sa | Sh | |||
| Bifurcaria bifurcata | August 2017 | Cantabria | 1.2/1.2 | 0.9/>19.9 | 0.3/0.3 | >19.9/>19.9 | 19.9/>19.2 | 6.5/>13.1 |
| May 2019 | Galicia | 0.4/0.4 | >24.5/>24.5 | 0.4/0.4 | >24.5/>24.5 | 12.2/12.2 | 6.1/6.1 | |
| May 2019 | Galicia | 3.2/6.4 | >25.6/>25.6 | 0.4/0.4 | 25.6/>25.6 | 12.8/12.8 | 6.4/6.4 | |
| December 2019 | Cantabria | 0.8/0.8 | 0.4/>23.9 | 0.3/0.3 | >23.9/>23.9 | 23.9/23.9 | 12.0/23.9 | |
| Ericaria selaginoides | August 2017 | Cantabria | 4.5/4.5 | >18.1/>18.1 | 0.3/0.3 | 2.3/4.5 | 2.3/2.3 | 2.3/4.5 |
| August 2019 | Galicia | 0.9/0.9 | 14.2/>14.2 | 0.2/0.2 | 3.6/3.6 | 3.6/3.6 | 7.1/7.1 | |
| Species | Life Stage | Month/Year | Region | Locality | Latitude Longitude | Littoral Zone |
|---|---|---|---|---|---|---|
| Dictyotales, Dictyotaceae | ||||||
| Dictyota dichotoma (Hudson) J. V. Lamouroux 1809 | Fertile and non-fertile | September 2019 | Cantabria | Comillas | 43°23′ N 4°17′ W | Subtidal (−1 m) |
| Fucales, Fucaceae | ||||||
| Ascophyllum nodosum (Linnaeus) Le Jolis 1863 | Fertile and non-fertile | August 2019 | Galicia | As Xubias, A Coruña | 43°20′ N 8°23′ W | Mid intertidal |
| Fucus ceranoides Linnaeus 1753 | Fertile and non-fertile | November 2019 | Galicia | O Burgo, Culleredo | 43°20′ N 8°21′ W | Mid intertidal |
| Fucus guiryi G. I. Zardi, K. R. Nicastro, E. S. Serrão & G. A. Pearson 2011 (=Fucus spiralis var. platycarpus (Thuret) Batters 1902) | Fertile and non-fertile | August 2017 | Cantabria | Comillas | 43°23′ N 4°17′ W | Upper intertidal |
| September 2019 | Cantabria | Comillas | ||||
| Fucus serratus Linnaeus 1753 | Fertile and non-fertile | November 2019 | Galicia | Esteiro, Muros | 42°47′ N 8°58′ W | Low intertidal |
| Fucus spiralis Linnaeus 1753 | Fertile and non-fertile | November 2019 | Galicia | As Xubias, A Coruña | 43°20′ N 8°23′ W | Upper intertidal |
| December 2019 | Cantabria | Comillas | 43°23′ N 4°17′ W | |||
| Fucus vesiculosus Linnaeus 1753 | Fertile and non-fertile | November | Galicia | Esteiro, Muros | 42°47′ N 8°58′ W | Mid intertidal |
| Pelvetia canaliculata (Linnaeus) Decaisne & Thuret 1845 | Fertile and non-fertile | August 2017 | Cantabria | Comillas | 43°23′ N 4°17′ W | Upper intertidal |
| Non-fertile | August 2019 | Galicia | Santa Cristina, Oleiros | 43°20′ N 8°22′ W | ||
| Fucales, Himanthaliaceae | ||||||
| Himanthalia elongata (Linnaeus) S. F. Gray 1821 | Non-fertile | May 2019 | Galicia | Barizo, Malpica | 43°19′ N 8°52′ W | Low intertidal and subtidal (−1 m) |
| Fertile | August 2019 | Galicia | Esteiro, Muros | 42°47′ N 8°58′ W | ||
| Fucales, Sargassaceae | ||||||
| Bifurcaria bifurcata R. Ross 1958 | Non-fertile | August 2017 | Cantabria | Trasvia, Comillas | 43°23′ N 4°17′ W | Mid intertidal |
| Non-fertile | May 2019 | Galicia | Portiño, Bens, A Coruña | 43°22′ N 8°26′ W | ||
| Fertile | May 2019 | Galicia | Portiño, Bens, A Coruña | 43°22′ N 8°26′ W | ||
| Fertile and non-fertile | Dec 2019 | Cantabria | Comillas | 43°23′ N 4°17′ W | ||
| Ericaria selaginoides (Linnaeus) Molinari & Guiry 2020 [=Carpodesmia tamariscifolia (Hudson) Orellana & Sansón 2019] [=Cystoseira tamariscifolia (Hudson) Papenfuss 1950] | Non-fertile | August 2017 | Cantabria | Trasvia, Comillas | 43°23′ N 4°17′ W | Low intertidal and subtidal (−1 m) |
| Non-fertile | August 2019 | Cantabria | Trasvia, Comillas | 43°23′ N 4°17′ W | ||
| Gongolaria baccata (S. G. Gmelin) Molinari & Guiry 2020 [=Treptacantha baccata (S. G. Gmelin) Orellana & Sansón 2019] [= Cystoseira baccata (S. G. Gmelin) P. C. Silva 1952] | Non-fertile | August 2017 | Cantabria | Trasvia, Comillas | 43°23′ N 4°17′ W | Low intertidal and subtidal (−1 m) |
| Halidrys siliquosa Linnaeus 1753 | Fertile and non-fertile | November 2019 | Galicia | Santa Cristina, Oleiros | 43°20′ N 8°22′ W | Subtidal (−1 m) |
| Sargassum muticum (Yendo) Fensholt 1955 | Fertile and non-fertile | May 2019 | Galicia | San Pedro de Veigue, Sada | 43°20′ N 8°17′ W | Subtidal (−1 m) |
| Laminariales, Alariaceae | ||||||
| Undaria pinnatifida (Harvey) Suringar 1873 | Non-fertile | May 2019 | Galicia | San Pedro de Veigue, Sada | 43°20′ N 8°17′ W | Low intertidal and subtidal (−1 m) |
| Laminariales, Laminariaceae | ||||||
| Saccharina latissima (Linnaeus) C. E. Lane, C. Mayes, Druehl & G. W. Saunders 2006 [= Laminaria saccharina (Linnaeus) J. V. Lamouroux 1813] | Non-fertile | August 2019 | Galicia | Esteiro, Muros | 42°47′ N 8°58′ W | Low intertidal and subtidal (−1 m) |
| Laminaria hyperborea (Gunnerus) Foslie 1885 | Non-fertile | August 2019 | Galicia | Esteiro, Muros | 42°47′ N 8°58′ W | Subtidal (−1 m) |
| Sphacelariales, Cladostephaceae | ||||||
| Cladostephus spongiosus (Hudson) C. Agardh 1817 | Non-fertile | September 2019 | Cantabria | Comillas | 43°23′ N 4°17′ W | Low intertidal |
| Tilopteridales, Phyllariaceae | ||||||
| Saccorhiza polyschides (Lightfoot) Batters 1902 | Non-fertile | May 2019 | Galicia | Portiño, Bens, A Coruña | 43°22′ N 8°26′ W | Low intertidal and subtidal (−1 m) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubiño, S.; Peteiro, C.; Aymerich, T.; Hortós, M. Brown Macroalgae (Phaeophyceae): A Valuable Reservoir of Antimicrobial Compounds on Northern Coast of Spain. Mar. Drugs 2022, 20, 775. https://doi.org/10.3390/md20120775
Rubiño S, Peteiro C, Aymerich T, Hortós M. Brown Macroalgae (Phaeophyceae): A Valuable Reservoir of Antimicrobial Compounds on Northern Coast of Spain. Marine Drugs. 2022; 20(12):775. https://doi.org/10.3390/md20120775
Chicago/Turabian StyleRubiño, Susana, César Peteiro, Teresa Aymerich, and Maria Hortós. 2022. "Brown Macroalgae (Phaeophyceae): A Valuable Reservoir of Antimicrobial Compounds on Northern Coast of Spain" Marine Drugs 20, no. 12: 775. https://doi.org/10.3390/md20120775