Effects of Diabetes and Voluntary Exercise on IgA Concentration and Polymeric Immunoglobulin Receptor Expression in the Submandibular Gland of Rats
Abstract
:1. Introduction
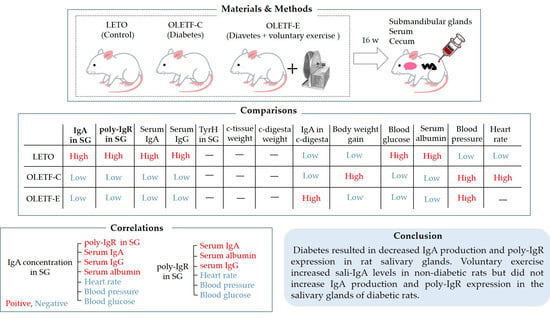
2. Materials and Methods
2.1. Animals
2.2. Sampling
2.3. Measurement of Blood Pressure and Heart Rate
2.4. Measurement of Blood Glucose Concentration
2.5. Measurement of Blood Albumin Concentration
2.6. Measurement of IgA Concentration
2.7. Measurement of Serum IgG Concentration
2.8. Measurement of Tyrosine Hydroxylase (TyrH) Concentration in SGs
2.9. Quantitative Real-Time Polymerase Chain Reaction (PCR) Analysis
2.10. SG Tissue Preparation for Hematoxylin–Eosin Staining
2.11. Statistical Analysis
3. Results
3.1. Body Weight Gain and SG Weight
3.2. Blood Glucose Concentration, Mean Blood Pressure, Serum Albumin Concentration, and Heart Rate
3.3. Histological Changes in SGs due to Diabetes Mellitus and Voluntary Exercise
3.4. c-Tissue Weight, c-Digesta Weight, and IgA Concentrations in c-Digesta
3.5. IgA and IgG Concentrations in Serum
3.6. IgA Concentration, Poly-IgR mRNA Expression Level, and TyrH Concentration in SGs
3.7. Correlations of IgA Concentration in the SGs with Different Parameters
3.8. Correlation of Poly-IgR mRNA Expression Level in the SGs with Different Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bouchi, R.; Sugiyama, T.; Goto, A.; Imai, K.; Ihana-Sugiyama, N.; Ohsugi, M.; Yamauchi, T.; Kadowaki, T.; Ueki, K. Retrospective nationwide study on the trends in first-line antidiabetic medication for patients with type 2 diabetes in Japan. J. Diabetes Investig. 2022, 13, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Bickett, A.; Tapp, H. Anxiety and diabetes: Innovative approaches to management in primary care. Exp. Biol. Med. 2016, 241, 1724–1731. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.M.; Cooper, M.E. Mechanisms of diabetic complications. Physiol. Rev. 2013, 93, 137–188. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.G.; Frier, B.M.; Ireland, J.T. Diabetes mellitus and infection. Postgrad. Med. J. 1985, 61, 233–237. [Google Scholar] [CrossRef]
- Berbudi, A.; Rahmadika, N.; Tjahjadi, A.I.; Ruslami, R. Type 2 Diabetes and its Impact on the Immune System. Curr. Diabetes Rev. 2020, 16, 442–449. [Google Scholar]
- Manoharan, A.; Winter, J. Tackling upper respiratory tract infections. Practitioner 2010, 254, 25–28. [Google Scholar]
- Tan, R.; Liu, B.; Zhao, C.; Yan, J.; Pan, T.; Zhou, M.; Qu, H. Nomogram for prediction of severe community-acquired pneumonia development in diabetic patients: A multicenter study. BMC Pulm. Med. 2022, 22, 403. [Google Scholar] [CrossRef]
- Carpenter, G.H. The secretion, components, and properties of saliva. Annu. Rev. Food. Sci. Technol. 2013, 4, 267–276. [Google Scholar] [CrossRef]
- Dawes, C.; Pedersen, A.M.; Villa, A.; Ekström, J.; Proctor, G.B.; Vissink, A.; Aframian, D.; McGowan, R.; Aliko, A.; Narayana, N.; et al. The functions of human saliva: A review sponsored by the World Workshop on Oral Medicine VI. Arch. Oral. Biol. 2015, 60, 863–874. [Google Scholar] [CrossRef]
- Humphrey, S.P.; Williamson, R.T. A review of saliva: Normal composition, flow, and function. J. Prosthet. Dent. 2001, 85, 162–169. [Google Scholar] [CrossRef]
- Otsuki, T.; Shimizu, K.; Iemitsu, M.; Kono, I. Chlorella intake attenuates reduced salivary SIgA secretion in kendo training camp participants. Nutr. J. 2012, 11, 103. [Google Scholar] [CrossRef]
- Hasegawa, H.; van Reit, E.; Kida, H. Mucosal immunization and adjuvants. Curr. Top. Microbiol. Immunol. 2015, 386, 371–380. [Google Scholar]
- Lehtonen, O.P.; Tenovuo, J.; Aaltonen, A.S.; Vilja, P. Immunoglobulins and innate factors of immunity in saliva of children prone to respiratory infections. Acta. Pathol. Microbiol. Immunol. Scand. C 1987, 95, 35–40. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Sugimoto, N.; Islam, R.; Hossain, M.E.; Sumiyoshi, E.; Katakura, M.; Shido, O. Salivary Immunoglobulin A Secretion and Polymeric Ig Receptor Expression in the Submandibular Glands Are Enhanced in Heat-Acclimated Rats. Int. J. Mol. Sci. 2020, 21, 815. [Google Scholar] [CrossRef]
- Sakaguchi, K.; Yokota, H.; Miyasho, T.; Maeda, N.; Nakamura, K.; Onaga, T.; Koiwa, M.; Matsuda, K.; Okamoto, M.; Hirayama, K.; et al. Polymeric immunoglobulin receptor expression and local immunoglobulin A production in bovine sublingual, submandibular and parotid salivary glands. Vet. J. 2013, 197, 291–296. [Google Scholar] [CrossRef]
- Chorzewski, M.; Orywal, K.; Sierpinska, T.; Golebiewska, M. Salivary protective factors in patients suffering from decompensated type 2 diabetes. Adv. Med. Sci. 2017, 62, 211–215. [Google Scholar] [CrossRef]
- Laing, S.J.; Gwynne, D.; Blackwell, J.; Williams, M.; Walters, R.; Walsh, N.P. Salivary IgA response to prolonged exercise in a hot environment in trained cyclists. Eur. J. Appl. Physiol. 2005, 93, 665–671. [Google Scholar] [CrossRef]
- Usui, T.; Yoshikawa, T.; Orita, K.; Ueda, S.Y.; Katsura, Y.; Fujimoto, S.; Yoshimura, M. Changes in salivary antimicrobial peptides, immunoglobulin A and cortisol after prolonged strenuous exercise. Eur. J. Appl. Physiol. 2011, 111, 2005–2014. [Google Scholar] [CrossRef]
- Sloan, C.A.; Engels, H.J.; Fahlman, M.M.; Yarandi, H.E.; Davis, J.E. Effects of exercise on S-IGA and URS in postmenopausal women. Int. J. Sports Med. 2013, 34, 81–86. [Google Scholar] [CrossRef]
- Chastin, S.F.M.; Abaraogu, U.; Bourgois, J.G.; Dall, P.M.; Darnborough, J.; Duncan, E.; Dumortier, J.; Pavón, D.J.; McParland, J.; Roberts, N.J.; et al. Effects of Regular Physical Activity on the Immune System, Vaccination and Risk of Community-Acquired Infectious Disease in the General Population: Systematic Review and Meta-Analysis. Sports Med. 2021, 51, 1673–1686. [Google Scholar] [CrossRef]
- Kurimoto, Y.; Saruta, J.; To, M.; Yamamoto, Y.; Kimura, K.; Tsukinoki, K. Voluntary exercise increases IgA concentration and polymeric Ig receptor expression in the rat submandibular gland. Biosci. Biotechnol. Biochem. 2016, 80, 2490–2496. [Google Scholar] [CrossRef] [PubMed]
- Proctor, G.B.; Carpenter, G.H.; Garrett, J.R. Sympathetic decentralization abolishes increased secretion of immunoglobulin A evoked by parasympathetic stimulation of rat submandibular glands. J. Neuroimmunol. 2000, 109, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Besnier, F.; Labrunée, M.; Richard, L.; Faggianelli, F.; Kerros, H.; Soukarié, L.; Bousquet, M.; Garcia, J.L.; Pathak, A.; Gales, C.; et al. Short-term effects of a 3-week interval training program on heart rate variability in chronic heart failure. A randomised controlled trial. Ann. Phys. Rehabil. Med. 2019, 62, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Sun, J.; Chen, H.; Zhang, H.; Zhang, Z.; Cai, D. Liraglutide prevents diabetes progression in prediabetic OLETF rats. Endocr. J. 2013, 60, 15–28. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Morozumi, T.; Takahashi, T.; Saruta, J.; Sakaguchi, W.; To, M.; Kubota, N.; Shimizu, T.; Kamata, Y.; Kawata, A.; et al. Effect of High Fat and Fructo-Oligosaccharide Consumption on Immunoglobulin A in Saliva and Salivary Glands in Rats. Nutrients 2021, 13, 1252. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, K.I. Blood Pressure Target in Type 2 Diabetes Mellitus. Diabetes. Metab. J. 2022, 46, 667–674. [Google Scholar] [CrossRef]
- Pretorius, E.; Lipinski, B.; Bester, J.; Vermeulen, N.; Soma, P. Albumin stabilizes fibrin fiber ultrastructure in low serum albumin type 2 diabetes. Ultrastruct. Pathol. 2013, 37, 254–257. [Google Scholar] [CrossRef]
- Singh, N. Diabetes, heart rate, and mortality. J. Cardiovasc. Pharmacol. Ther. 2002, 7, 117–129. [Google Scholar] [CrossRef]
- Oikawa, J.; Ukawa, S.; Ohira, H.; Kawamura, T.; Wakai, K.; Ando, M.; Hata, A.; Tamakoshi, A. Diabetes Mellitus is Associated with Low Secretion Rates of Immunoglobulin A in Saliva. J. Epidemiol. 2015, 25, 470–474. [Google Scholar] [CrossRef]
- Yamamoto, Y.; To, M.; Hayashi, T.; Shimizu, T.; Kamata, Y.; Saruta, J.; Takahashi, T.; Tsukinoki, K. Intake of indigestible carbohydrates influences IgA response and polymeric Ig receptor expression in the rat submandibular gland. Br. J. Nutr. 2015, 113, 1895–1902. [Google Scholar] [CrossRef]
- Carpenter, G.H.; Proctor, G.B.; Garrett, J.R. Preganglionic parasympathectomy decreases salivary SIgA secretion rates from the rat submandibular gland. J. Neuroimmunol. 2005, 160, 4–11. [Google Scholar] [CrossRef]
- Hordern, M.D.; Dunstan, D.W.; Prins, J.B.; Baker, M.K.; Singh, M.A.; Coombes, J.S. Exercise prescription for patients with type 2 diabetes and pre-diabetes: A position statement from Exercise and Sport Science Australia. J. Sci. Med. Sport 2012, 15, 25–31. [Google Scholar] [CrossRef]
- Trochimiak, T.; Hübner-Woźniak, E. Effect of exercise on the level of immunoglobulin a in saliva. Biol. Sport 2012, 29, 255–261. [Google Scholar] [CrossRef]
- Shoji, K.; Michihata, N.; Miyairi, I.; Matsui, H.; Fushimi, K.; Yasunaga, H. Recent epidemiology of Pneumocystis pneumonia in Japan. J. Infect. Chemother. 2020, 26, 1260–1264. [Google Scholar] [CrossRef]
- Carpenter, G.H.; Proctor, G.B.; Anderson, L.C.; Zhang, X.S.; Garrett, J.R. Immunoglobulin A secretion into saliva during dual sympathetic and parasympathetic nerve stimulation of rat submandibular glands. Exp. Physiol. 2000, 85, 281–286. [Google Scholar] [CrossRef]
- Sinski, M.; Jedrusik, P.; Lewandowski, J. Why Not All Hypertensive Patients Are Tachycardic at Rest? Curr. Pharm. Des. 2017, 23, 4626–4634. [Google Scholar] [CrossRef]
- Burgi, K.; Cavalleri, M.T.; Alves, A.S.; Britto, L.R.; Antunes, V.R.; Michelini, L.C. Tyrosine hydroxylase immunoreactivity as indicator of sympathetic activity: Simultaneous evaluation in different tissues of hypertensive rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R264–R271. [Google Scholar] [CrossRef]
- Vinik, A.I.; Erbas, T. Diabetic autonomic neuropathy. Handb. Clin. Neurol. 2013, 117, 279–294. [Google Scholar]
- Franco, C.C.S.; Prates, K.V.; Previate, C.; Moraes, A.M.P.; Matiusso, C.C.I.; Miranda, R.A.; de Oliveira, J.C.; Tófolo, L.P.; Martins, I.P.; Barella, L.F.; et al. Glibenclamide treatment blocks metabolic dysfunctions and improves vagal activity in monosodium glutamate-obese male rats. Endocrine 2017, 56, 346–356. [Google Scholar] [CrossRef]
- Mori, A.; Maruyama, T.; Ohashi, N.; Shibuya, T.; Sakai, K.; Tatebe, H.; Inoue, H.; Kato, T.; Okuno, M. Impaired autonomic function in type 2 diabetic patients during upper gastrointestinal endoscopy. J. Gastroenterol. 2008, 43, 603–608. [Google Scholar] [CrossRef]
- Xuan, Y.L.; Wang, Y.; Xue, M.; Hu, H.S.; Cheng, W.J.; Li, X.R.; Yin, J.; Yang, N.; Yan, S.H. In rats the duration of diabetes influences its impact on cardiac autonomic innervations and electrophysiology. Auton. Neurosci. 2015, 189, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Kwon, K.J.; Lee, E.J.; Kim, M.K.; Kim, S.Y.; Kim, J.N.; Kim, J.O.; Kim, H.J.; Kim, H.Y.; Han, J.S.; Shin, C.Y.; et al. Diabetes augments cognitive dysfunction in chronic cerebral hypoperfusion by increasing neuronal cell death: Implication of cilostazol for diabetes mellitus-induced dementia. Neurobiol. Dis. 2015, 73, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Bayram, R.O.; Özdemir, H.; Emsen, A.; Türk Dağı, H.; Artaç, H. Reference ranges for serum immunoglobulin (IgG, IgA, and IgM) and IgG subclass levels in healthy children. Turk. J. Med. Sci. 2019, 49, 497–505. [Google Scholar] [CrossRef] [PubMed]
- de la Torre, M.C.; Torán, P.; Serra-Prat, M.; Palomera, E.; Güell, E.; Vendrell, E.; Yébenes, J.C.; Torres, A.; Almirall, J. Serum levels of immunoglobulins and severity of community-acquired pneumonia. BMJ Open Respir. Res. 2016, 3, e000152. [Google Scholar] [CrossRef] [PubMed]
- Zambad, S.P.; Upganlawar, S.P.; Umathe, S.N. A synergistic decline in humoral and cellular immunity of diabetic mice on exposure to polluted air. Indian J. Physiol. Pharmacol. 1999, 43, 474–478. [Google Scholar]
- Lucas, R.M.; Ponsonby, A.L.; Dear, K. Mid-life stress is associated with both up- and down-regulation of markers of humoral and cellular immunity. Stress 2007, 10, 351–361. [Google Scholar] [CrossRef]
- Muthukumar, A.R.; Jolly, C.A.; Zaman, K.; Fernandes, G. Calorie restriction decreases proinflammatory cytokines and polymeric Ig receptor expression in the submandibular glands of autoimmune prone (NZB x NZW) F1 mice. J. Clin. Immunol. 2000, 20, 354–361. [Google Scholar] [CrossRef]
- Liu, T.W.; Park, Y.M.; Holscher, H.D.; Padilla, J.; Scroggins, R.J.; Welly, R.; Britton, S.L.; Koch, L.G.; Vieira-Potter, V.J.; Swanson, K.S. Physical Activity Differentially Affects the Cecal Microbiota of Ovariectomized Female Rats Selectively Bred for High and Low Aerobic Capacity. PLoS ONE 2015, 10, e0136150. [Google Scholar] [CrossRef]
- Nieman, D.C.; Pence, B.D. Exercise immunology: Future directions. J. Sport Health. Sci. 2020, 9, 432–445. [Google Scholar] [CrossRef]
- Drago-Serrano, M.E.; Godínez-Victoria, M.; Lara-Padilla, E.; Resendiz-Albor, A.A.; Reyna-Garfias, H.; Arciniega-Martínez, I.M.; Kormanovski-Kovsova, A.; Campos-Rodriguez, R. Moderate exercise enhances expression of SIgA in mouse ileum. Int. J. Sports Med. 2012, 33, 1020–1025. [Google Scholar] [CrossRef]
- Rosa, L.; Teixeira, A.; Lira, F.; Tufik, S.; Mello, M.; Santos, R. Moderate acute exercise (70% VO2 peak) induces TGF-β, α-amylase and IgA in saliva during recovery. Oral Dis. 2014, 20, 186–190. [Google Scholar] [CrossRef]
- Dworacki, G.; Urazayev, O.; Bekmukhambetov, Y.; Iskakova, S.; Frycz, B.A.; Jagodziński, P.P.; Dworacka, M. Thymic emigration patterns in patients with type 2 diabetes treated with metformin. Immunology 2015, 146, 456–469. [Google Scholar] [CrossRef]






| IgA Concentrations in SGs | |||
|---|---|---|---|
| r * | p | n | |
| poly-IgR § mRNA expression level in SGs | 0.76 | 0.001 | 15 |
| IgA concentration in serum | 0.68 | 0.005 | 15 |
| Albumin concentration in serum | 0.63 | 0.01 | 15 |
| IgG concentration in serum | 0.60 | 0.02 | 15 |
| c-digesta ‡ weight | 0.16 | 0.6 | 15 |
| IgA concentration in c-digesta | 0.056 | 0.8 | 15 |
| Heart rate | −0.76 | 0.001 | 15 |
| Mean blood pressure | −0.62 | 0.01 | 15 |
| Blood glucose concentration | −0.59 | 0.02 | 15 |
| c-tissue # weight | −0.44 | 0.1 | 15 |
| TyrH † concentration in SGs | −0.11 | 0.7 | 15 |
| Poly-IgR mRNA Expression Levels in SGs | |||
|---|---|---|---|
| r * | p | n | |
| IgA concentration in serum | 0.81 | 0.0002 | 15 |
| Albumin concentration in serum | 0.73 | 0.002 | 15 |
| IgG concentration in serum | 0.64 | 0.01 | 15 |
| IgA concentration in c-digesta | 0.094 | 0.7 | 15 |
| c-digesta ‡ weight | 0.051 | 0.9 | 15 |
| Heart rate | −0.75 | 0.001 | 15 |
| Mean blood pressure | −0.64 | 0.01 | 15 |
| Blood glucose concentration | −0.64 | 0.01 | 15 |
| c-tissue # weight | −0.37 | 0.2 | 15 |
| TyrH † concentration in SGs | −0.10 | 0.7 | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Yamamoto, Y.; Hidaka, K.; Wada-Takahashi, S.; Takahashi, S.-s.; Morozumi, T.; Kubota, N.; Saita, M.; Saruta, J.; Sakaguchi, W.; et al. Effects of Diabetes and Voluntary Exercise on IgA Concentration and Polymeric Immunoglobulin Receptor Expression in the Submandibular Gland of Rats. Medicina 2023, 59, 789. https://doi.org/10.3390/medicina59040789
Park J, Yamamoto Y, Hidaka K, Wada-Takahashi S, Takahashi S-s, Morozumi T, Kubota N, Saita M, Saruta J, Sakaguchi W, et al. Effects of Diabetes and Voluntary Exercise on IgA Concentration and Polymeric Immunoglobulin Receptor Expression in the Submandibular Gland of Rats. Medicina. 2023; 59(4):789. https://doi.org/10.3390/medicina59040789
Chicago/Turabian StylePark, Jaebum, Yuko Yamamoto, Kouki Hidaka, Satoko Wada-Takahashi, Shun-suke Takahashi, Toshiya Morozumi, Nobuhisa Kubota, Makiko Saita, Juri Saruta, Wakako Sakaguchi, and et al. 2023. "Effects of Diabetes and Voluntary Exercise on IgA Concentration and Polymeric Immunoglobulin Receptor Expression in the Submandibular Gland of Rats" Medicina 59, no. 4: 789. https://doi.org/10.3390/medicina59040789