Maternal Gestational Diabetes Is Associated with High Risk of Childhood Overweight and Obesity: A Cross-Sectional Study in Pre-School Children Aged 2–5 Years
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Study Design
2.3. Statistical Analysis
3. Results
3.1. Sociodemographic and Anthropometric Parameters and Perinatal Outcomes of the Study Population
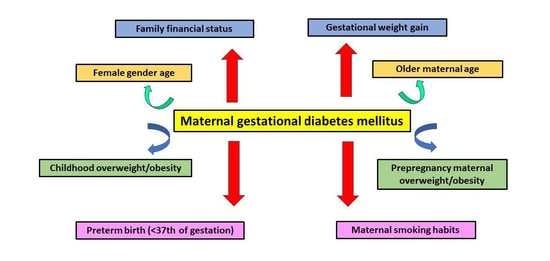
3.2. Maternal Gestational Diabetes Mellitus in Relation to Sociodemographic and Anthropometric Parameters and Perinatal Outcomes of the Study Population
3.3. Multivariate Regression Analysis for Gestational Diabetes Mellitus Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Voerman, E.; Santos, S.; Patro Golab, B.; Amiano, P.; Ballester, F.; Barros, H.; Bergström, A.; Charles, M.A.; Chatzi, L.; Chevrier, C.; et al. Maternal body mass index, gestational weight gain, and the risk of overweight and obesity across childhood: An individual participant data meta-analysis. PLoS Med. 2019, 16, e1002744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemmingsson, E. Early Childhood Obesity Risk Factors: Socioeconomic Adversity, Family Dysfunction, Offspring Distress, and Junk Food Self-Medication. Curr. Obes. Rep. 2018, 7, 204–209. [Google Scholar] [CrossRef] [Green Version]
- Weihrauch-Blüher, S.; Wiegand, S. Risk Factors and Implications of Childhood Obesity. Curr. Obes. Rep. 2018, 7, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Drozdz, D.; Alvarez-Pitti, J.; Wójcik, M.; Borghi, C.; Gabbianelli, R.; Mazur, A.; Herceg-Čavrak, V.; Lopez-Valcarcel, B.G.; Brzeziński, M.; Lurbe, E.; et al. Obesity and Cardiometabolic Risk Factors: From Childhood to Adulthood. Nutrients 2021, 13, 4176. [Google Scholar] [CrossRef] [PubMed]
- Branca, F.; Nikogosian, H.; Lobstein, T. (Eds.) The Challenge of Obesity in the WHO European Region and the Strategies for Response; WHO Regional Office for Europe: Copenhagen, Denmark, 2007; Available online: https://www.euro.who.int/__data/assets/pdf_file/0008/98243/E89858.pdf (accessed on 21 September 2022).
- Cattaneo, A.; Monasta, L.; Stamatakis, E.; Lioret, S.; Castetbon, K.; Frenken, F.; Manios, Y.; Moschonis, G.; Savva, S.; Zaborskis, A.; et al. Overweight and obesity in infants and pre-school children in the European Union: A review of existing data. Obes. Rev. 2010, 11, 389–398. [Google Scholar] [CrossRef]
- Jones, R.E.; Jewell, J.; Saksena, R.; Ramos Salas, X.; Breda, J. Overweight and Obesity in Children under 5 Years: Surveillance Opportunities and Challenges for the WHO European Region. Front. Public Health 2017, 5, 58. [Google Scholar] [CrossRef] [Green Version]
- Manios, Y.; Costarelli, V.; Kolotourou, M.; Kondakis, K.; Tzavara, C.; Moschonis, G. Prevalence of obesity in preschool Greek children, in relation to parental characteristics and region of residence. BMC Public Health 2007, 7, 178. [Google Scholar] [CrossRef] [Green Version]
- Saravanan, P.; Diabetes in Pregnancy Working Group; Maternal Medicine Clinical Study Group; Royal College of Obstetricians and Gynaecologists UK. Gestational diabetes: Opportunities for improving maternal and child health. Lancet Diabetes Endocrinol. 2020, 8, 793–800. [Google Scholar] [CrossRef]
- Dunn, A.B.; Hanson, L.; VandeVusse, L.; Leslie, S. Through the Microbial Looking Glass: Premature Labor, Preeclampsia, and Gestational Diabetes: A Scoping Review. J. Perinat. Neonatal Nurs. 2019, 33, 35–51. [Google Scholar] [CrossRef]
- Alejandro, E.U.; Mamerto, T.P.; Chung, G.; Villavieja, A.; Gaus, N.L.; Morgan, E.; Pineda-Cortel, M.R.B. Gestational Diabetes Mellitus: A Harbinger of the Vicious Cycle of Diabetes. Int. J. Mol. Sci. 2020, 21, 5003. [Google Scholar] [CrossRef]
- Schwartz, N.; Nachum, Z.; Green, M.S. Risk factors of gestational diabetes mellitus recurrence: A meta-analysis. Endocrine 2016, 53, 662–771. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Hu, J.; Liu, Y.; Luo, B.; Lei, A. Risk of type 2 diabetes mellitus after gestational diabetes mellitus: A systematic review & meta-analysis. Indian J. Med. Res. 2021, 154, 62–77. [Google Scholar] [CrossRef]
- Bellamy, L.; Casas, J.P.; Hingoranim, A.D.; Williams, D. Type 2 diabetes mellitus after gestational diabetes: A systematic review and meta-analysis. Lancet 2009, 373, 1773–1779. [Google Scholar] [CrossRef] [PubMed]
- Abu-Heija, A.T.; Al-Bash, M.R.; Al-Kalbani, M.A. Effects of maternal age, parity and pre-pregnancy body mass index on the glucose challenge test and gestational diabetes mellitus. J. Taibah Univ. Med. Sci. 2017, 12, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Najafi, F.; Hasani, J.; Izadi, N.; Hashemi-Nazari, S.S.; Namvar, Z.; Mohammadi, S.; Sadeghi, M. The effect of prepregnancy body mass index on the risk of gestational diabetes mellitus: A systematic review and dose-response meta-analysis. Obes. Rev. 2019, 20, 472–486. [Google Scholar] [CrossRef] [PubMed]
- Najafi, F.; Hasani, J.; Izadi, N.; Hashemi-Nazari, S.S.; Namvar, Z.; Shamsi, H.; Erfanpoor, S. Risk of gestational diabetes mellitus by pre-pregnancy body mass index: A systematic review and meta-analysis. Diabetes Metab. Syndr. 2021, 15, 102181. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Z.; Mo, M.; Muyiduli, X.; Wang, S.; Li, M.; Jiang, S.; Wu, Y.; Shao, B.; Shen, Y.; et al. The association of gestational diabetes mellitus with fetal birth weight. J. Diabetes Complicat. 2018, 32, 635–642. [Google Scholar] [CrossRef]
- Logan, K.M.; Gale, C.; Hyde, M.J.; Santhakumaran, S.; Modi, N. Diabetes in pregnancy and infant adiposity: Systematic review and meta-analysis. Arch. Dis. Child Fetal Neonatal. Ed. 2017, 102, F65–F72. [Google Scholar] [CrossRef] [Green Version]
- Kamana, K.; Shakya, S.; Zhang, H. Gestational diabetes mellitus and macrosomia: A literature review. Ann. Nutr. Metab. 2015, 66, 14–20. [Google Scholar] [CrossRef]
- Rozance, P.J.; Hay, W.W. Hypoglycemia in newborn infants: Features associated with adverse outcomes. Biol. Neonate 2006, 90, 74–86. [Google Scholar] [CrossRef]
- Franks, P.W.; Looker, H.C.; Kobes, S.; Touger, L.; Tataranni, P.A.; Hanson, R.L.; Knowler, W.C. Gestational glucose tolerance and risk of type 2 diabetes in young Pima Indian offspring. Diabetes 2006, 55, 460–465. [Google Scholar] [CrossRef] [Green Version]
- Burguet, A. Long-term outcome in children of mothers with gestational diabetes. Diabetes Metab. 2010, 36, 682–694. [Google Scholar] [CrossRef]
- Moore, T.R. Fetal exposure to gestational diabetes contributes to subsequent adult metabolic syndrome. Am. J. Obstet. Gynecol. 2010, 202, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Scholtens, D.M.; Kuang, A.; Lowe, L.P.; Hamilton, J.; Lawrence, J.M.; Lebenthal, Y.; Brickman, W.J.; Clayton, P.; Ma, R.C.; McCance, D.; et al. HAPO Follow-up Study Cooperative Research Group; HAPO Follow-Up Study Cooperative Research Group. Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study (HAPO FUS): Maternal Glycemia and Childhood Glucose Metabolism. Diabetes Care 2019, 42, 381–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leybovitz-Haleluya, N.; Wainstock, T.; Landau, D.; Sheiner, E. Maternal gestational diabetes mellitus and the risk of subsequent pediatric cardiovascular diseases of the offspring: A population-based cohort study with up to 18 years of follow up. Acta Diabetol. 2018, 55, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Franzago, M.; Fraticelli, F.; Stuppia, L.; Vitacolonna, E. Nutrigenetics, epigenetics and gestational diabetes: Consequences in mother and child. Epigenetics 2019, 14, 215–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantzorou, M.; Papandreou, D.; Vasios, G.K.; Pavlidou, E.; Antasouras, G.; Psara, E.; Taha, Z.; Poulios, E.; Giaginis, C. Exclusive breastfeeding for at least four months is associated with a higher prevalence of overweight and obesity in mothers and their children after 2-5 years from delivery. Nutrients 2022, 14, 3599. [Google Scholar] [CrossRef]
- Papandreou, D.; Mantzorou, M.; Tyrovolas, S.; Pavlidou, E.; Antasouras, G.; Psara, E.; Poulios, E.; Vasios, G.K.; Giaginis, C. Pre-Pregnancy Excess Weight Association with Maternal Sociodemographic, Anthropometric and Lifestyle Factors and Maternal Perinatal Outcomes. Nutrients 2022, 14, 3810. [Google Scholar] [CrossRef] [PubMed]
- Sellen, D. Physical Status: The Use and Interpretation of Anthropometry. Report of a WHO Expert Committee. WHO Technical Report Series No. 854. Pp. 452. (WHO, Geneva, 1995.) Swiss Fr 71.00. J. Biosoc. Sci. 1998, 30, 135–144. [Google Scholar] [CrossRef]
- Centers for Disease Control Prevention. National Health and Nutrition Examination Survey (NHANES): Anthropometry Procedures Manual; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2007. Available online: https://www.cdc.gov/nchs/data/nhanes/nhanes_07_08/manual_an.pdf (accessed on 11 September 2022).
- Cole, T.J.; Bellizzi, M.C.; Flegal, K.M.; Dietz, W.H. Establishing a standard definition for child overweight and obesity worldwide: International survey. BMJ 2000, 320, 1240–1245. [Google Scholar] [CrossRef] [Green Version]
- WHO. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications. Part I: Diagnosis and Classification of Diabetes Mellitus; WHO: Geneva, Switzerland, 1999; pp. 1–59.
- Spinelli, A.; Buoncristiano, M.; Kovacs, V.A.; Yngve, A.; Spiroski, I.; Obreja, G.; Starc, G.; Pérez, N.; Rito, A.I.; Kunešová, M.; et al. Prevalence of Severe Obesity among Primary School Children in 21 European Countries. Obes. Facts. 2019, 12, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Hassapidou, M.; Daskalou, E.; Tsofliou, F.; Tziomalos, K.; Paschaleri, A.; Pagkalos, I.; Tzotzas, T. Prevalence of overweight and obesity in preschool children in Thessaloniki, Greece. Hormones 2015, 14, 615–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anastasiou, E.; Farmakidis, G.; Gerede, A.; Goulis, D.G.; Koukkou, E.; Kourtis, A.; Mamopoulos, A.; Papadimitriou, K.; Papadopoulos, V.; Stefos, T. Clinical practice guidelines on diabetes mellitus and pregnancy: ΙI. Gestational diabetes mellitus. Hormones 2020, 19, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Wang, H.; Li, W.; Wang, L.; Li, N.; Qiao, Y.; Zhang, T.; Li, J.; Yu, Z.; Hu, G.; et al. Maternal insulin resistance and maternal β-cell function during pregnancy for offspring overweight before 2 years of age among women with gestational diabetes. Pediatr. Obes. 2022, 15, e12995. [Google Scholar] [CrossRef]
- Lowe, W.L.; Lowe, L.P.; Kuang, A.; Catalano, P.M.; Nodzenski, M.; Talbot, O.; Tam, W.H.; Sacks, D.A.; McCance, D.; Linder, B.; et al. Maternal glucose levels during pregnancy and childhood adiposity in the Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study. Diabetologia 2019, 62, 598–610. [Google Scholar] [CrossRef] [Green Version]
- Lowe, W.L.; Scholtens, D.M.; Lowe, L.P.; Kuang, A.; Nodzenski, M.; Talbot, O.; Catalano, P.M.; Linder, B.; Brickman, W.J.; Clayton, P.; et al. HAPO Follow-up Study Cooperative Research Group. Association of Gestational Diabetes With Maternal Disorders of Glucose Metabolism and Childhood Adiposity. JAMA 2018, 320, 1005–1016. [Google Scholar] [CrossRef] [Green Version]
- Choi, M.J.; Yu, J.; Choi, J. Maternal Pre-Pregnancy Obesity and Gestational Diabetes Mellitus Increase the Risk of Childhood Obesity. Children 2022, 9, 928. [Google Scholar] [CrossRef]
- Ardıç, C.; Çolak, S.; Uzun, K.; Salı, G.; Aydemir, T.; Telatar, G. Maternal Gestational Diabetes and Early Childhood Obesity: A Retrospective Cohort Study. Child Obes. 2020, 16, 579–585. [Google Scholar] [CrossRef]
- Bawah, A.T.; Seini, M.M.; Abaka-Yawason, A.; Alidu, H.; Nanga, S. Leptin, resistin and visfatin as useful predictors of gestational diabetes mellitus. Lipids Health Dis. 2019, 18, 221. [Google Scholar] [CrossRef] [Green Version]
- Seneviratne, S.N.; Rajindrajith, S. Fetal programming of obesity and type 2 diabetes. World J. Diabetes 2022, 13, 482–497. [Google Scholar] [CrossRef]
- Zhu, Q.; Yang, X.; Zhang, Y.; Shan, C.; Shi, Z. Role of the Gut Microbiota in the Increased Infant Body Mass Index Induced by Gestational Diabetes Mellitus. mSystems 2022, 7, e0046522. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Sharma, A.J.; Callaghan, W.M. Gestational diabetes and childhood obesity: What is the link? Curr. Opin. Obstet. Gynecol. 2012, 24, 376–381. [Google Scholar] [CrossRef] [Green Version]
- Ward, Z.J.; Long, M.W.; Resch, S.C.; Giles, C.M.; Cradock, A.L.; Gortmaker, S.L. Simulation of Growth Trajectories of Childhood Obesity into Adulthood. N. Engl. J. Med. 2017, 377, 2145–2153. [Google Scholar] [CrossRef]
- Simmonds, M.; Llewellyn, A.; Owen, C.G.; Woolacott, N. Predicting adult obesity from childhood obesity: A systematic review and meta-analysis. Obes. Rev. 2016, 17, 95–107. [Google Scholar] [CrossRef] [Green Version]
- Weihrauch-Blüher, S.; Schwarz, P.; Klusmann, J.H. Childhood obesity: Increased risk for cardiometabolic disease and cancer in adulthood. Metabolism 2019, 92, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Reilly, J.J.; Kelly, J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: Systematic review. Int. J. Obes. 2011, 35, 891–898. [Google Scholar] [CrossRef] [Green Version]
- Santos, S.; Voerman, E.; Amiano, P.; Barros, H.; Beilin, L.J.; Bergström, A.; Charles, M.A.; Chatzi, L.; Chevrier, C.; Chrousos, G.P.; et al. Impact of maternal body mass index and gestational weight gain on pregnancy complications: An individual participant data meta-analysis of European, North American and Australian cohorts. BJOG 2019, 126, 984–995. [Google Scholar] [CrossRef]
- Yue, S.; Vu Thi, V.T.K.; Dung, L.P.; Nhu, B.T.H.; Kestelyn, E.; Thuan, D.T.; Thanh, L.Q.; Hirst, J.E. Clinical consequences of gestational diabetes mellitus and maternal obesity as defined by asian BMI thresholds in Viet Nam: A prospective, hospital-based, cohort study. BMC Pregnancy Childbirth 2022, 22, 195. [Google Scholar] [CrossRef]
- Petry, C.J.; Hughes, I.A.; Ong, K.K. Increased basal insulin sensitivity in late pregnancy in women carrying a male fetus: A cohort study. Biol. Sex Differ. 2022, 13, 20. [Google Scholar] [CrossRef]
- Kedziora, S.M.; Obermayer, B.; Sugulle, M.; Herse, F.; Kräker, K.; Haase, N.; Langmia, I.M.; Müller, D.N.; Staff, A.C.; Beule, D.; et al. Placental Transcriptome Profiling in Subtypes of Diabetic Pregnancies Is Strongly Confounded by Fetal Sex. Int. J. Mol. Sci. 2022, 23, 15388. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Cruz, M.; Palatnik, A.; Olivier-Van Stichelen, S. O-GlcNAc transferase contributes to sex-specific placental deregulation in gestational diabetes. Placenta 2022, 131, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mandò, C.; Anelli, G.M.; Novielli, C.; Panina-Bordignon, P.; Massari, M.; Mazzocco, M.I.; Cetin, I. Impact of Obesity and Hyperglycemia on Placental Mitochondria. Oxid. Med. Cell Longev. 2018, 2018, 2378189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fattuoni, C.; Mandò, C.; Palmas, F.; Anelli, G.M.; Chiara Novielli, C.; Laudicina, E.P.; Savasi, V.M.; Barberini, L.; Dessì, A.; Pintus, R.; et al. Preliminary metabolomics analysis of placenta in maternal obesity. Placenta 2018, 61, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Hooks, S.K.; Abiodun-Ojo, O.; Noah, A.I.; Hill, A.V.; Perez-Patron, M.J.; Menon, R.; Taylor, B.D. Evaluating the Impact of Fetal Sex on Gestational Diabetes Mellitus Following Interaction with Maternal Characteristics. Reprod. Sci. 2022, in press. [CrossRef]
- Seghieri, G.; Di Cianni, G.; Gualdani, E.; De Bellis, A.; Franconi, F.; Francesconi, P. The impact of fetal sex on risk factors for gestational diabetes and related adverse pregnancy outcomes. Acta Diabetol. 2022, 59, 633–639. [Google Scholar] [CrossRef]
- Mandò, C.; Calabrese, S.; Mazzocco, M.I.; Novielli, C.; Anelli, G.M.; Antonazzo, P.; Cetin, I. Sex specific adaptations in placental biometry of overweight and obese women. Placenta 2016, 38, 1–7. [Google Scholar] [CrossRef]
- Billionnet, C.; Mitanchez, D.; Weill, A.; Nizard, J.; Alla, F.; Hartemann, A.; Jacqueminet, S. Gestational diabetes and adverse perinatal outcomes from 716,152 births in France in 2012. Diabetologia 2017, 60, 636–644. [Google Scholar] [CrossRef] [Green Version]
- Hedderson, M.M.; Ferrara, A.; Sacks, D.A. Gestational diabetes mellitus and lesser degrees of pregnancy hyperglycemia: Association with increased risk of spontaneous preterm birth. Obstet. Gynecol. 2003, 102, 850–856. [Google Scholar] [CrossRef]
- Li, Y.; Ren, X.; He, L.; Li, J.; Zhang, S.; Chen, W. Maternal age and the risk of gestational diabetes mellitus: A systematic review and meta-analysis of over 120 million participants. Diabetes Res. Clin. Pract. 2020, 162, 108044. [Google Scholar] [CrossRef]
- Wendland, E.M.; Pinto, M.E.; Duncan, B.B.; Belizán, J.M.; Schmidt, M.I. Cigarette smoking and risk of gestational diabetes: A systematic review of observational studies. BMC Pregnancy Childbirth 2008, 8, 53. [Google Scholar] [CrossRef] [Green Version]
- Bar-Zeev, Y.; Haile, Z.T.; Chertok, I.A. Association Between Prenatal Smoking and Gestational Diabetes Mellitus. Obstet. Gynecol. 2020, 135, 91–99. [Google Scholar] [CrossRef]
- Kim, M.K.; Han, K.; You, S.Y.; Kwon, H.S.; Yoon, K.H.; Lee, S.H. Prepregnancy smoking and the risk of gestational diabetes requiring insulin therapy. Sci. Rep. 2020, 10, 13901. [Google Scholar] [CrossRef] [PubMed]
- Morales-Suárez-Varela, M.; Peraita-Costa, I.; Perales-Marín, A.; Llopis-Morales, A. Risk of Gestational Diabetes Due to Maternal and Partner Smoking. Int. J. Environ. Res. Public Health 2022, 19, 925. [Google Scholar] [CrossRef] [PubMed]
- da Silva, M.D.C.M.; Assis, A.M.O.; Pinheiro, S.M.C.; de Oliveira, L.P.M.; da Cruz, T.R.P. Breastfeeding and maternal weight changes during 24 months post-partum: A cohort study. Matern. Child Nutr. 2015, 11, 780–791. [Google Scholar] [CrossRef]
- Arenz, S.; Rückerl, R.; Koletzko, B.; von Kries, R. Breast-feeding and childhood obesity--a systematic review. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 1247–1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, J.; Liu, L.; Zhu, Y.; Huang, G.; Wang, P.P. The association between breastfeeding and childhood obesity: A meta-analysis. BMC Public Health 2014, 14, 1267. [Google Scholar] [CrossRef] [Green Version]
- Harder, T.; Bergmann, R.; Gerd Kallischnigg, G.; Plagemann, A. Duration of breastfeeding and risk of overweight: A meta-analysis. Am. J. Epidemiol. 2005, 162, 397–403. [Google Scholar] [CrossRef] [Green Version]
- Amir, L.H.; Donath, S. A systematic review of maternal obesity and breastfeeding intention, initiation and duration. BMC Pregnancy Childbirth 2007, 7, 9. [Google Scholar] [CrossRef] [Green Version]
- Ramji, N.; Challa, S.; Murphy, P.A.; Quinlan, J.; Crane, J.M.G. A comparison of breastfeeding rates by obesity class. J. Matern. Fetal Neonatal Med. 2018, 31, 3021–3026. [Google Scholar] [CrossRef] [PubMed]
- Jirakittidul, P.; Panichyawat, N.; Chotrungrote, B.; Mala, A. Prevalence and associated factors of breastfeeding in women with gestational diabetes in a university hospital in Thailand. Int. Breastfeed. J. 2019, 14, 34. [Google Scholar] [CrossRef] [Green Version]
- Zaragoza-Martí, A.; Ruiz-Ródenas, N.; Herranz-Chofre, I.; Sánchez-SanSegundo, M.; de la Cruz Serrano Delgado, V.; Hurtado-Sánchez, J.A. Adherence to the Mediterranean Diet in Pregnancy and Its Benefits on Maternal-Fetal Health: A Systematic Review of the Literature. Front. Nutr. 2022, 9, 813942. [Google Scholar] [CrossRef] [PubMed]
- Farella, I.; Francesca Miselli, F.; Campanozzi, A.; Grosso, F.A.; Laforgia, N.; Baldassarre, M.E. Mediterranean Diet in Developmental Age: A Narrative Review of Current Evidences and Research Gaps. Children 2022, 9, 906. [Google Scholar] [CrossRef] [PubMed]

| Characteristics (n = 5348) | Descriptive Statistics |
|---|---|
| Maternal age (mean ± SD; years) at the time of pregnancy | 33.7 ± 4.7 |
| Children age (mean ± SD; years) at the time of study | 4.06 ± 1.06 |
| Children gender (n, %) | |
| Male | 2639 (49.3%) |
| Female | 2709 (50.7%) |
| Children BMI status at 2–5 years old (n, %) | |
| Normal weight | 4033 (75.4%) |
| Overweight | 878 (16.4%) |
| Obese | 437 (8.2%) |
| Maternal pre-pregnancy BMI status (n, %) | |
| Underweight and normal weight | 4148 (77.6%) |
| Overweight | 936 (17.5%) |
| Obese | 264 (4.9%) |
| Maternal education level (mean ± SD; years) | 14.6 ± 2.8 |
| Family economic status (n, %) | |
| Low | 2449 (45.8%) |
| Medium | 2440 (45.6%) |
| High | 459 (8.6%) |
| Maternal smoking habits (n, %) | |
| No smokers | 3984 (74.5%) |
| Smokers | 1364 (25.5%) |
| Parity (n, %) | |
| Nulliparity | 3193 (59.7%) |
| Multiparity | 2155 (40.3%) |
| Maternal gestational weigh gain (mean ± SD; Kg) | 13.8 ± 6.1 |
| Preterm birth (<37th week; n, %) | |
| No | 3739 (69.9%) |
| Yes | 1609 (30.1%) |
| Maternal gestational diabetes (n, %) | |
| No | 5052 (94.5%) |
| Yes | 235 (5.5%) |
| Maternal gestational hypertension (n, %) | |
| No | 5129 (95.9%) |
| Yes | 219 (4.1%) |
| Exclusive breastfeeding (n, %) | |
| No | 2689 (50.2%) |
| Yes | 2659 (49.8%) |
| Childbirth weight (n, %) | |
| Low newborn weight (<2500 g) | 436 (8.2%) |
| Normal newborn weight (2500–4000 g) | 4564 (85.3%) |
| High newborn weight (>4000 g) | 348 (6.5%) |
| Characteristics (n = 5348) | Gestational Diabetes Mellitus | ||
|---|---|---|---|
| No (94.5%) | Yes (5.5%) | p-Value | |
| Maternal age (mean ± SD; years) at the time of pregnancy | 33.4 ± 4.7 | 36.1 ± 4.8 | p < 0.0001 |
| Children gender (n, %) | p = 0.0012 | ||
| Male | 2520 (49.9) | 119 (40.2) | |
| Female | 2532 (50.1) | 177 (59.8) | |
| Children BMI status (n, %) | p < 0.0001 | ||
| Normal weight | 3890 (77.0) | 143 (48.3) | |
| Overweight | 795 (15.7) | 83 (28.0) | |
| Obese | 367 (7.3) | 70 (23.7) | |
| Maternal pre-pregnancy BMI status (n, %) | p = 0.0155 | ||
| Underweight and normal weight | 3934 (77.9) | 214 (72.3) | |
| Overweight and obese | 1118 (22.1) | 82 (27.7) | |
| Maternal education level (mean ± SD; years) | 14.6 ± 2.8 | 14.7 ± 2.8 | p = 0.6765 |
| Family economic status (n, %) | p = 0.0065 | ||
| Low | 2300 (45.5) | 149 (50.3) | |
| Medium | 2304 (45.6) | 136 (46.0) | |
| High | 448 (8.9) | 11 (3.7) | |
| Maternal smoking habits (n, %) | p = 0.0049 | ||
| No smokers | 3784 (74.9) | 200 (67.6) | |
| Smokers | 1268 (25.1) | 96 (32.4) | |
| Parity (n, %) | p = 0.0552 | ||
| Nulliparity | 3032 (60.0) | 161 (54.4) | |
| Multiparity | 2020 (40.0) | 135 (45.6) | |
| Maternal gestational weigh gain (mean ± SD; Kg) | 13.0 ± 5.0 | 13.8 ± 6.2 | p = 0.0323 |
| Preterm birth (<37th week, n, %) | p = 0.0002 | ||
| No | 3560 (70.5) | 179 (60.5) | |
| Yes | 1492 (29.5) | 117 (39.5) | |
| Maternal gestational hypertension (n, %) | p = 0.1647 | ||
| No | 4839 (95.8) | 290 (98.0) | |
| Yes | 213 (4.2) | 6 (2.0) | |
| Exclusive breastfeeding (n, %) | p = 0.0656 | ||
| No | 2527 (50.0) | 162 (54.8) | |
| Yes | 2525 (50.0) | 134 (45.3) | |
| Childbirth weight (n, %) | p = 0.3115 | ||
| Low newborn weight (<2500 g) | 405 (8.0) | 31 (10.5) | |
| Normal newborn weight (2500–4000 g) | 4321 (85.5) | 243 (82.1) | |
| High newborn weight (>4000 g) | 326 (7.5) | 22 (7.4) | |
| Characteristics | Gestational Diabetes Mellitus | |
|---|---|---|
| OR * (95% CI **) | p-Value | |
| Children BMI status (Normal weight/overweight or obese) | 2.13 (1.94–2.31) | p = 0.0006 |
| Children gender (Male/female) | 1.27 (0.88–1.69) | p = 0.0050 |
| Maternal age (Below/over mean value) at the time of pregnancy | 1.29 (0.92–1.64) | p = 0.0027 |
| Maternal pre-pregnancy BMI status (Underweight and normal weigh/overweight and obese) | 1.30 (0.79–1.92) | p = 0.0326 |
| Maternal education level (Below/over mean value) | 0.97 (0.22–1.87) | p = 0.8502 |
| Family economic status (Low or medium/high) | 0.89 (0.24–1.71) | p = 0.2147 |
| Smoking habits (No/yes) | 1.54 (1.16–1.97) | p = 0.0204 |
| Parity (Nulliparity/multiparity) | 1.26 (0.69–1.80) | p = 0.2813 |
| Maternal gestational weigh gain (Below/over mean value) | 1.40 (0.61–2.12) | p = 0.3172 |
| Preterm birth (No/yes) | 1.77 (1.41–2.16) | p = 0.0135 |
| Maternal gestational hypertension (No/yes) | 1.14 (0.43–1.96) | p = 0.3804 |
| Exclusive breastfeeding (No/yes) | 1.31 (0.86–1.89) | p = 0.0932 |
| Childbirth weight (Low or normal/high) | 1.19 (0.52–1.91)) | p = 0.4311 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mantzorou, M.; Papandreou, D.; Pavlidou, E.; Papadopoulou, S.K.; Tolia, M.; Mentzelou, M.; Poutsidi, A.; Antasouras, G.; Vasios, G.K.; Giaginis, C. Maternal Gestational Diabetes Is Associated with High Risk of Childhood Overweight and Obesity: A Cross-Sectional Study in Pre-School Children Aged 2–5 Years. Medicina 2023, 59, 455. https://doi.org/10.3390/medicina59030455
Mantzorou M, Papandreou D, Pavlidou E, Papadopoulou SK, Tolia M, Mentzelou M, Poutsidi A, Antasouras G, Vasios GK, Giaginis C. Maternal Gestational Diabetes Is Associated with High Risk of Childhood Overweight and Obesity: A Cross-Sectional Study in Pre-School Children Aged 2–5 Years. Medicina. 2023; 59(3):455. https://doi.org/10.3390/medicina59030455
Chicago/Turabian StyleMantzorou, Maria, Dimitrios Papandreou, Eleni Pavlidou, Sousana K. Papadopoulou, Maria Tolia, Maria Mentzelou, Antigoni Poutsidi, Georgios Antasouras, Georgios K. Vasios, and Constantinos Giaginis. 2023. "Maternal Gestational Diabetes Is Associated with High Risk of Childhood Overweight and Obesity: A Cross-Sectional Study in Pre-School Children Aged 2–5 Years" Medicina 59, no. 3: 455. https://doi.org/10.3390/medicina59030455